0102
Magnetic resonance recording of local neuronal firings (mrLNF) in the human brain: A proof of concept1Radiology, New York University, New York, NY, United States, 2Neurology, New York University, New York, NY, United States
Synopsis
Neurons are firing when emitting action potentials to communicate with each other. Action potentials generate fast electric currents (~2ms duration) across membrane and slow ones (~10–100ms) at postsynaptic side. These currents generate electric and magnetic fields detectable by scalp EEG and MEG, respectively. They detect the fields relatively far away (~20mm) from firing sources and are only sensitive to slow, easily-synchronized postsynaptic currents. Here we propose a new approach termed as magnetic resonance recording of local neuronal firings (mrLNF) that has a very high temporal resolution (0.25ms) and can non-invasively detect fast and slow neuronal currents at the firing sources.
INTRODUCTION
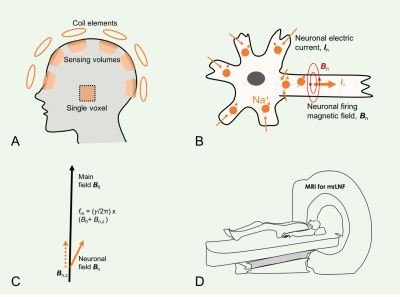
A neuron is said to fire when emitting an action potential, a cellular electrical event traveling down the axon and inducing neurochemical changes that allow communication between neurons. These traveling action potentials generate a fast-changing electric current (~2ms in duration) across the cell membrane as well as a slow-changing one (~10–100ms) on the postsynaptic side. Neuronal currents generate electric and magnetic fields detectable by scalp electroencephalography (EEG) and magnetoencephalography (MEG), respectively.1,2 Signals from EEG and MEG have contributed to our understanding of an array of neurological disorders such as epilepsy,3 brain injury,4 and cognitive impairment.5 However, scalp EEG and MEG detect the fields relatively far away (~20mm) from the firing sources, sensitive only to the slow, easily-synchronized postsynaptic currents, and provide insight restricted to the collective behavior of neuronal activities within a relatively large anatomic region.1,6,7 To improve spatial localization of neuronal firings, fast magnetic resonance imaging (MRI) approaches (e.g., EPI and spiral combined with parallel imaging and/or compressed sensing) have been proposed, but these tools are only able to detect the slow postsynaptic currents due to limits of temporal resolution (~40–100ms).2,8 Here, we propose a novel approach we have termed magnetic resonance recording of local neuronal firings (mrLNF, Fig. 1), that can non-invasively detect both fast and slow neuronal currents based on high temporal resolution (0.25ms) and can spatially localize the firing sources. This technique has the potential to extend detection to deep brain regions (hippocampus, corpus calosum, and thalamus), and even peripheral nerves throughout the body.THEORY
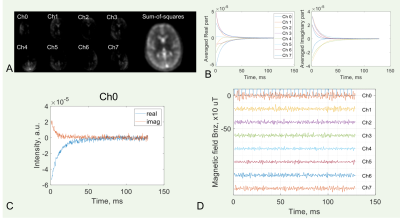
Neuronal current In(r, t) at location r and time t in the brain generates a local magnetic field Bn(r, t) which alters resonance frequency f0=(γ/2π)B0 by an amount of fn(r, t) (Eq. 1). This alteration is naturally encoded in free induction decay (FID) s(t) during MR data acquisition at an investigation volume (or voxel) ΔV of magnetization density ρ(r) and effective transverse relaxation constant T2*(r) (Eq. 2). A phase variation is calculated at sampling interval Δt after the correction for T2* decay (Eq. 3). Finally, the calculated frequency fn is scaled via Eq. 4 to the firing field z-component Bn,z. The firing-induced magnetic field, although very small (~10-4 µT) above the scalp,1,6,7 is large enough (~1600 µT) in and around firing neurons (~4–10 µm in diameter) to alter local resonance frequency by up to 68.1 kHz for the proton (1H) or 18.0 kHz for the sodium (23Na) MRI. Fig. 2 summarizes the data processing of this method.Eq.1a. fm(r, t) = f0 + fn(r, t),
Eq.1b. fn(r, t) = (γ/2π)Bn,z(r, t).
Eq.2a. s(t) = ∑ΔV ρ(r)·exp(-t/T2*)·exp(-jφ(r,t))dr,
Eq.2b. φ(r, t) = φ(r, TE) + 2π∑TEt fn(r, τ)dτ.
Eq.3a. fn(t) = dφ/dt - Δf0 ≈ phase[Δs(t)·Δs*(t+Δt)]/Δt - Δf0,
Eq.3b. Δs(t) Ξ (s(t) - sc(t))/σ + s0.
Eq.4. Bn,z(t) = (2π/γ)fn(t).
METHODS
A retrospective study is performed to test the proposed idea. Healthy subjects (n=7, age 36.8±14.8, male/female 3/4) were included and provided the informed consent. FID signals were acquired at resting state during X-Frequency adjustment for sodium (23Na) MRI at 3T (Prisma, Siemens), with a custom-built 8-channel dual-tuned (1H-23Na) head array coil.9 The investigation-volume was defined on individual coil images. The FID acquisition had a readout time 128ms (sampling interval 0.25ms) at TE/TR=1/200ms and continuously repeated 128 times. Data were analyzed using MATLAB R2020a (MathWorks, Natick, MA). T2* decay sc(t) was estimated by averaging multiple FIDs. The noise σ was estimated from Δs(t). Constant s0 was set to 5.RESULTS
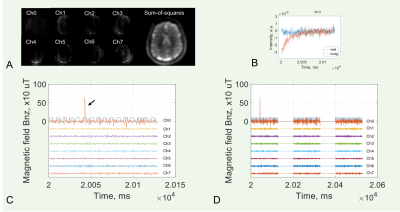
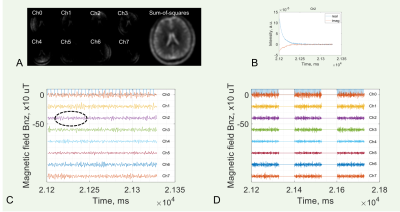
The measured neuron-firing magnetic field across all subjects studied was ±750 µT, falling within the theoretical range (Figs. 3,4). The field arising from the fast action potentials was measured as 0.75–2.0 ms in duration and -722.3–678.9 µT in strength (Fig. 3c,d), while the field relating to the slow postsynaptic current was measured at 10–43 ms in duration and -10.0–10.0 µT in strength (Fig. 4c,d). The spatial distribution of neuronal firings was observed in both left and right hemispheres.DISCUSSION
The results show feasibility of our proposed idea based on the physical basis of localized quantum sensing of neuronal currents. As intrinsic micro quantum sensors, nuclear spins (Na+ in this study) present throughout intra- and extracellular spaces and locate in and around the firing sources. The frequency calculation in Eq. 3a is prone to noise interference and could be improved. The investigation volume, passively defined by the coil sensitivity in this study, can be actively defined at any location in the brain or potentially any part of the body when using single voxel excitation. Strength of the main magnetic field B0 would not necessarily be as high as 3T if proton (1H) MRI is employed, and lower field strength of 1.5 or 0.5T could accommodate this technique, raising the possibility of low-field, portable MRI even at point of care.CONCLUSION
Human subject resting-state data presented here supports the proposed idea that magnetic resonance can be used to non-invasively record neuronal firings of both fast action potentials and slow postsynaptic currents in the brain by analyzing continuous acquisition of FID signals. This idea, in principle, has the potential to be extended from brain cortex to deep brain structures as well as other anatomic body parts, and could be applied at variable field strengths.Acknowledgements
This work was financially supported by the General Research Fund of the Department of Radiology. This work was also performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407-420.
2. Hennig J, Kiviniemi V, Riemenschneider B, et al. 15 Years MR-encephalography. MAGMA. 2020 Oct 20. Epub ahead of print.
3. Rosenow F, Klein KM, Hamer HM. Non-invasive EEG evaluation in epilepsy diagnosis. Expert Rev Neurother. 2015 Apr;15(4):425-44.
4. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci. 2017;20:327–339.
5. Henin S, Borges H, Shankar A, Sarac C, Melloni L, Friedman D, Flinker A, Parra LC, Buzsaki G, Devinsky O, Liu A. Closed-loop acoustic stimulation enhances sleep oscillations but not memory performance. eNeuro 11 October 2019, 6 (6) ENEURO.0306-19.2019.
6. Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005;29:65–88.
7. Boudurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultra-weak, transient magnetic field changes. Magn Reson Med. 2002;47(6):1052-1058.
8. Huang J. Detecting neuronal currents with MRI: a human study. Magn Reson Med. 2014;71(2):756-762.
9. Lakshmanan K, Brown R, Madelin G, Qian Y, Boada F, Wiggins GC. An eight-channel sodium/proton coil for brain MRI at 3 T. NMR Biomed. 2018 Feb;31(2):10.1002/nbm.3867.
Figures