0092
R2* mapping of the whole brain with 0.8 mm isotropic resolution at 7T in less than 7 minutes
Arun Joseph1,2,3, Tobias Kober4,5,6, and Tom Hilbert4,5,6
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
T2*-weighted imaging is an important diagnostic tool to evaluate normal and pathological tissues due to its sensitivity to iron deposition. This comes with high sensitivity to magnetic field inhomogeneities within a voxel, resulting in susceptibility artifacts. These artefacts are largely reduced at higher resolutions; high-resolution protocols however lead to clinically unfeasible scan times with multi-echo gradient echo sequences. Here, we propose a compressed sensing multi-echo GRE acquisition for 7T to obtain 0.8 mm isotropic R2* maps of the whole brain in <7 minutes and with substantially reduced artefacts. Preliminary qualitative and quantitative validations are performed on healthy subjects.
Introduction
Quantitative R2* mapping can be an important predictor of iron deposition in the brain and other organs of the human body1. The accurate estimation of R2* maps are highly dependent on multi-echo T2*-weighted acquisitions which are sensitive to local magnetic field inhomogeneities caused by susceptibility differences (e.g. at air-tissue boundaries above the nasal cavity) resulting in geometric distortion or signal voids2. One approach to mitigate these artifacts is the reduction of intra-voxel dephasing through acquisitions with smaller voxel size3. Imaging at high magnetic field strength provides improved signal-to-noise ratios which can be used to achieve higher resolutions and thus mitigate these artefacts. However, the acquisition time increases drastically with higher resolution leading to impractical experimental protocols.Here, we propose to use a compressed sensing (CS) multi-echo GRE sequence not to accelerate the acquisition but to trade the gained acquisition time for higher resolution and thus significantly reduced susceptibility artifacts.
Methods
Four subjects were scanned at 7T (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) using a 1-channel transmit and 32-channel receive head coil (Nova Medical, Wilmington, USA) after written informed consent was obtained. A reference measurement with isotropic resolution of 1.6 mm was performed using a product GRE sequence with GRAPPAx2 acceleration resulting in an acquisition time of 6.02 min. A Cartesian spiral-phyllotaxis undersampling scheme4 was implemented in a prototype GRE sequence. This prototype was then used to acquire two additional datasets.- An acquisition that has the same resolution but a 5-fold CS acceleration (TA 1.42 min).
- An acquisition that uses a 5-fold acceleration but with a smaller voxel size (0.8 mm) to both approximately match the acquisition time (TA 6.39 min) of the reference and reduce susceptibility artifacts.
Image reconstructions of all measurements were performed inline on the scanner. While GRAPPAx2 measurements used the product reconstruction, CS reconstructions were performed using a prototype iterative algorithm5-7 with Haar wavelet regularization. The complex coil sensitivity maps for the CS reconstruction were estimated using the ESPIRiT algorithm8. The inline reconstruction time for the CS reconstruction was ~5 minutes for one multi-echo dataset. All post-processing and analysis of images were performed using Matlab (MathWorks, USA). R2* maps were generated for each dataset by performing a mono-exponential log-linear fit.
A region of interest analysis was performed on seven white matter (WM) and gray matter (GM) structures (frontal-WM/GM, occipital-WM/GM, parietal-WM/GM, and corpus collosum) using the prototype MorphoBox9 segmentation algorithm on the MP2RAGE images. The resulting label maps were copied into the space of the R2* maps using rigid registrations10. The median values from all regions were extracted using the obtained masks. Finally, the mean and standard deviation across subjects for the different brain structures and acquisitions were compared in a bar plot.
Results and Discussion
Figure 1 shows T2*-weighted images of the fifth echo obtained from the GRAPPAx2 and CS acquisitions. The CS images show similar contrast to GRAPPAx2. Besides the improvement in resolution, a drastic reduction of susceptibility artifacts can be seen in the CS acquisition at higher resolution of 0.8 mm (see also red arrows in Figure 1). Figure 2 shows the R2* maps generated from the multi-echo GRE acquisitions. The R2* maps obtained from GRAPPAx2 and CS acquisitions were found to be qualitatively comparable to each other. The R2* maps from CS acquisition with 0.8 mm isotropic resolution had fewer susceptibility artifacts. Figure 3 shows R2* maps from the transversal view for GRAPPAx2 and CS acquisitions. The effect of high-resolution CS acquisition is demonstrated through clear delineation of mid brain structures such as the substantia nigra. Figure 4 shows the distribution of median values over three subjects for the different regions of interest and acquisitions. The median values obtained from the R2* maps for GRAPPAx2 and CS reconstruction were found to be similar. The median values obtained from CS acquisition with 0.8 mm isotropic resolution was lower than the 1.6 mm isotropic GRAPPAx2 and CS acquisitions. Figure 5 animates a comparison between GRAPPAx2 and CS acquisitions with 1.6 mm and 0.8 mm resolution to highlight difference due to resolution. Although both acquisitions have a similar scan time of around 6 mins, a clear difference in image quality can be observed.Conclusion
We implemented CS acquisitions based on Cartesian spiral-phyllotaxis readout scheme at 7T to generate 0.8 mm isotropic R2* maps of the whole brain at a scan time of <7 mins. The preliminary data indicated that CS reconstruction at higher resolution provided consistent data with drastically reduced susceptibility artifacts and good qualitative information of the midbrain structures, which enables its use in future clinical studies.Acknowledgements
No acknowledgement found.References
- Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005;106(4):1460-5.
- Chavhan GB, Babyn PS, Thomas B, et al. Principles, Techniques, and Applications of T2*-based MR Imaging and Its Special Applications. RadioGraphics. 2009;29 (5): 1433–1449.
- Petersa AM, Brookesa MJ, Hoogenraadb FG, et al. T2* measurements in human brain at 1.5, 3 and 7 T. Magn Reson Imag. 2007; 25:748–753.
- Mussard E, Hilbert T, Forman C, et al. Accelerated MP2RAGE imaging using Cartesian phyllotaxis readout and compressed sensing reconstruction. Magn Reason Med. 2020; 84(4):1881-1894.
- Forman C, Piccini D, Grimm R, et al. High-resolution 3D whole-heart coronary MRA: a study on the combination of data acquisition in multiple breath-holds and 1D residual respiratory motion compensation. MAGMA. 2014;27(5):435-43.
- Wetzl J, Forman C, Wintersperger BJ, et al. High-resolution dynamic CE-MRA of the thorax enabled by iterative TWIST reconstruction. Magn Reson Med. 2017;77(2):833-840.
- Ma LE, Markl M, Chow K, et al. Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k-space reordering, and inline reconstruction. Magn Reson Med. 2019;81(6):3675-3690.
- Uecker M, Lai P, Murphy MJ, et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 2014;71(3):990-1001.
- Schmitter D, Roche A, Maréchal B et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. Neuroimage Clin. 2014; 7:7-17.
- Klein S, Staring M, Murphy K, et al. "Elastix: a toolbox for intensity based medical image registration," IEEE Transactions on Medical Imaging 2010; 29(1):196-205.
Figures
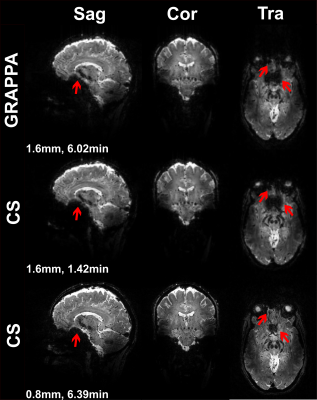
Figure 1:
Magnitude images of the fifth echo obtained from T2*-weighted multi-echo
GRE acquisitions for GRAPPAx2 and compressed sensing (CS) reconstructions with 1.6
mm and 0.8 mm isotropic resolution.
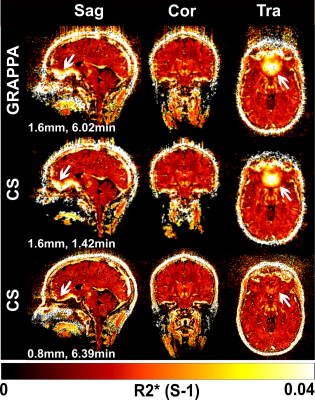
Figure 2:
R2* maps from different views obtained from GRAPPAx2 and compressed
sensing reconstructions with 1.6 mm and 0.8 mm isotropic resolutions.
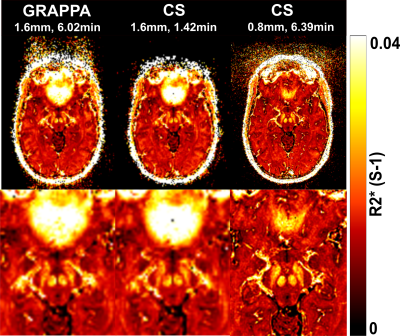
Figure 3:
Transversal R2* maps of the midbrain region obtained from GRAPPAx2 and compressed
sensing reconstructions with 1.6 mm and 0.8 mm isotropic resolutions. Zoomed
images of these R2* maps are displayed in the bottom row.
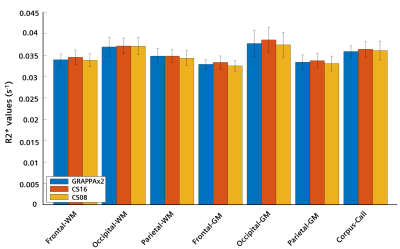
Figure 4:
Regional values of R2* maps
averaged across four healthy subjects. The error bars indicate the standard
deviation across subjects.
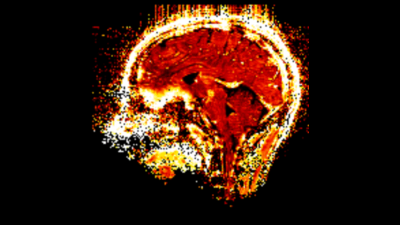
Figure 5:
Comparison of sagittal R2*
maps obtained from GRAPPAx2 and CS acquisition with 1.6 mm and 0.8 mm
resolution.