0090
Deep learning based acceleration of multi-contrast MRI examinations by acquiring contrast and sharing inter-contrast structure information1GE Healthcare, Bangalore, India
Synopsis
We propose a method to accelerate multi-contrast MRI examination for a subject. We accelerate typically longer scans of MR exam such as FLAIR-T1, FLAIR-T2 by only acquiring its contrast information and sharing structure information from a reference scan from the same exam which is fully sampled (typically with the lowest acquisition time e.g. T2FSE). The resulting view-shared images have systematic artifacts which are then removed by a deep learning module trained on more than 200 cases. We observed high quality reconstruction (SSIM>0.9) for both healthy control and pathology cases with acceleration factors of 2x and 3x for FLAIR-T1 and FLAIR-T2.
Introduction
MRI examinations typically involves acquisition of multiple contrasts (such as T2FSE, FLAIR-T2, FLAIR-T1 etc.). In general practice, each contrast is accelerated independently as required. Individual acceleration schemes primarily comprise of methods to reconstruct an under-sampled k-space acquisition using compressed sensing1 and/or, more recently, DL-based methods2 which rely on intelligent methods to fill in k-space data which was not acquired. Besides this, another effective approach to synthesize multi-contrast images with shorter acquisition times from MDME data is MAGiC3 with known limitations. Keyhole imaging techniques4 have been used to accelerate dynamic MR imaging. It has some known limitations5 such as insufficient resolution of structures at higher acceleration levels.We present a method which enables acquisition of high-resolution multiple contrast data in an MRI examination. We exploit the k-space redundancy across scan data within an exam to reduce total acquisition time. A fully sampled reference scan acquisition (typically T2FSE, due to its low acquisition time) can be used to accelerate a FLAIR scan. For the accelerated FLAIR images, only the central region of k-space is acquired. This ensures true contrast for the fast FLAIR images and avoids contrast transfer or synthesis. The high frequency structure information for the fast FLAIR images are borrowed from fully sampled reference scan by simple k-space grafting technique. The artifacts due to this grafting are removed by training a deep learning module to identify and remove these grafting artifacts. Whereas traditional keyhole imaging concept has been limited predominantly to accelerate dynamic and quantitative MR imaging4,7, we extend the concept to cross-contrast anatomical imaging with deep learning enhancements to address artifacts arising from cross contrast structure sharing. We demonstrate the performance on clinical grade scans. Additionally, pathology cases were evaluated to ensure reliable performance of the method in clinical settings.
Methods
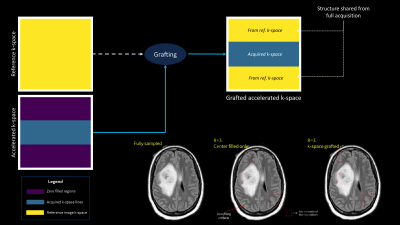
Sharing structure acquiring contrastA fully sampled reference scan is obtained, T2FSE in our case (fast scan with high SNR). Only the central region around the k-space is acquired for the fast FLAIR scans. The number of k-space lines acquired around the center is decided by the acceleration factor. For example, an acceleration of 2x for an image which acquires 256 phase encode (PE) lines in k-space, would require acquisition of 128 PE lines around the center. The remaining outer k-space is grafted from the reference image k-space. This is motivated from the fact that outer k-space predominantly contains high frequency structure information which is common in the two contrast scans. This process and its efficacy is shown in Figure-1.
Deep learning module
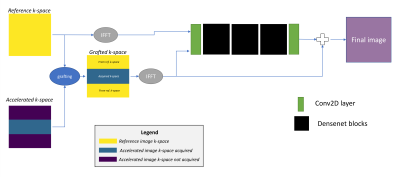
The structure sharing between the reference and fast scan creates grafting artifacts which can be seen in Figure-1. A deep learning network is trained to reduce this artifact. The network is trained on the reference and the grafted accelerated scans to predict grafting artifact and then remove them. Network architecture: 3 cascaded blocks of dense networks8 with convolutional2D layers at each end. Each dense block has 5 layers. The network was trained with MAE and SSIM6 as loss. Networks were trained for 2x and 3x acceleration levels for FLAIR-T1 and FLAIR-T2. Refer Figure-2 for training workflow.
Data
The training sets consisted of 230 and 217 datasets for FLAIR-T1 and FLAIR-T2 respectively with corresponding T2FSE as reference scans. The trained network was tested on 7 FLAIR T1 and FLAIR T2 datasets each (healthy controls-5, pathology cases-2). Images were reviewed with two experienced radiologists for diagnostic/clinical equivalence of accelerated images.
Results
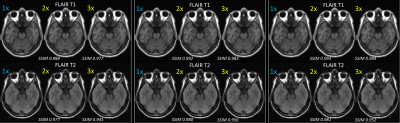
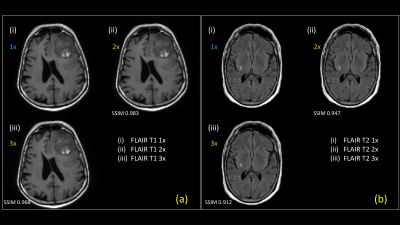
Results of an exam where FLAIR-T1 and FLAIR-T2 were accelerated (2x and 3x) using a reference T2FSE scan are shown in Figure-3. Images were acquired on a 1.5T GE Signa Creator MR system. T2FSE, FLAIR-T1 and FLAIR-T2 had in-plane resolution of 0.9375mm isotropic with #averages of 2, 2 and 1 respectively. Slice thickness/slice spacing of T2FSE, FLAIR-T1 and FLAIR-T2 are 4.5mm/6mm, 4.5mm/6mm and 5mm/6mm respectively. Our method has the capability to deal with accelerated images acquired at different resolution than the reference scan. The consolidated metrics comparing the accelerated reconstruction with fully sampled reconstruction for 7 test cases are shown in Figure-4. Accelerated FLAIR-T1 and FLAIR-T2 images for a pathology test case are shown in Figure-5. Both radiologists found accelerated images preserved features for diagnostic equivalence for both FLAIR-T1 and FLAIR-T2.Discussion
In this work we present a method to accelerate longer (and clinically regularly used) sequences in a typical MR examination ensuring:- Contrast preservation while accelerating: Contrast is a critical part of a MR exam. In the proposed method, enough lines are acquired around the central k-space region to ensure that the MRI contrast of interest is acquired rather than recovering contrast using DL or other methods.
- Use high frequency structure sharing for sharper images: The k-space representation was leveraged to share high frequency structure across contrasts. Training the DL with the reference and fast grafted image provides DL good priors on corrections. This ensures better reconstruction.
Acknowledgements
No acknowledgement found.References
[1] Lustig, M., et al. (2008). IEEE signal processing magazine, 25(2), 72-82.
[2] Sandino, Christopher M., et al. IEEE Signal Processing Magazine 37, no. 1 (2020): 117-127.
[3] Tanenbaum, Lawrence N., et al. AJNR 38, no. 6 (2017): 1103-1110.
[4] Van Vaals, Joop J., et al. JMRI 3, no. 4 (1993): 671-675.
[5] Bishop, Jonathan E et al. JMRI 7, no. 4 (1997): 716-723.
[6] Wang, Zhou, et al. IEEE TIP 13, no. 4 (2004): 600-612.
[7] Zaitsev M., et al., MRM, no. 45 (2001):109-117.
[8] Huang, Gao, et al., In Proceedings of the IEEE CVPR (2017), pp. 4700-4708.
Figures