0089
Single-shot simultaneous diffusion and T2 mapping based on overlapping-echo detachment planar imaging1Department of Electronic Science, Xiamen University, Xiamen, China
Synopsis
A single-shot simultaneous diffusion and T2 mapping method is developed based on overlapping-echo detachment planar imaging (DT2M-OLED) incorporating with deep-learning reconstruction. The method makes it possible to realize simultaneous diffusion and T2 mapping in around one hundred milliseconds for the first time, and owns an advantage in resisting motion artifacts. The accuracy of the proposed method was verified by numerical experiment and in vivo rat brain experiment. Dynamic diffusion and T2 mapping on rat recovering from deep anesthesia was also conducted to test the capability of the proposed method in real-time imaging.
Introduction
Apparent diffusion coefficient (ADC) and T2 maps have been increasingly used to cooperatively estimate various treatment modalities for diagnosis (e.g. cerebral ischemia, prostate cancer). However, the collaborative usage of ADC and T2 maps is hampered due to the time limitation in clinical application, and also suffer from motion artifacts. Quantitative MRI methods based on overlapping-echo detachment (OLED) are able to deliver parametric maps in single-shot.1-3 Compared to conventional image reconstruction method using separation algorithm,1 deep-learning based method exhibits better performance in image details recovering and higher robustness to non-ideal radio-frequency (B1) field.2 In this work, we developed a single-shot synchronized diffusion and T2 mapping based on DT2M-OLED, which is capable of gaining reliable and self-registered diffusion and T2 maps within 120 ms for the first time.Methods
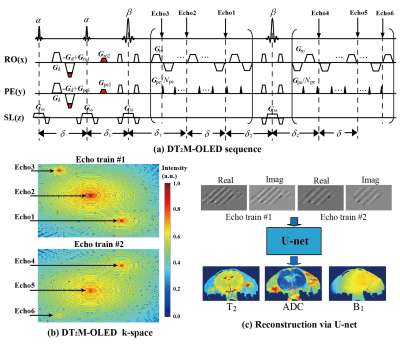
In the DT2M-OLED sequence (Fig. 1(a)), six echoes with two diffusion weighting and three T2 weighting (Fig. 1(b)) are generated by two excitation pulses α (α flip angle = 60°), refocusing pulses β (β flip angle =180°), diffusion gradient Gd and echo-shifting gradients (Gro1, Gro2, Gpe1 and Gpe2) in two echo trains.U-net was employed for T2 and ADC maps reconstruction. The training dataset were the real parts and imaginary parts of the overlapping-echo images from two echo trains (matrix size of 96×96) zero-filled to 160×160 and then randomly cropped into 64 × 64 matrix as the input of the network (Fig1. (c)). Totally 6000 and 1000 samples were used for training and testing, respectively. B1 inhomogeneity and Gaussian noise were added to the training data for accounting for non-ideal experimental conditions. Adam optimizer was applied with an initial learning rate of 0.0001.
The technique was tested on numerical and rat brain experiments. Same sequence parameters were used in DT2M-OLED acquisition for both samples. For the numerical experiment, parametric maps were also reconstructed by separation algorithm to demonstrate the advantage of deep learning reconstruction.
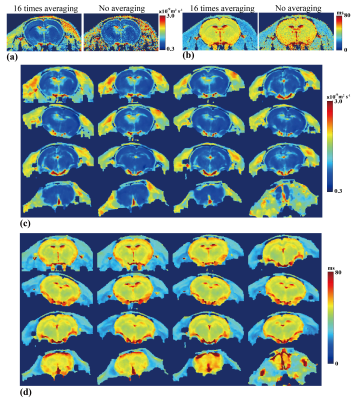
In-vivo experiments were conducted on a 7T Varian MRI system. Two healthy Wistar rats were used for brain scanning with DT2M-OLED and spin-echo echo-planar imaging (SE-EPI). Resolution = 0.3 × 0.3 × 2.0 mm3. One b = 800 s/mm2 for both sequences and one b = 0 image for SE-EPI. SE-EPI acquired multiple TEs (TE=50/80/110/140 ms) for T2 mapping. Reference B1 map was calculated from EPI images with flip angle of 60° and 120°, respectively. Condition A: Rat under deep anesthetic, 16 times signal averaging for both sequences, TR = 3 s. Condition B: Anesthetic gas was closed, temporal DT2M-OLED data were acquired every 3 s during the period of rat recovering from deep sleep without signal averaging. The acquisition time of one DT2M-OLED scan is 114 ms. Before temporal DT2M-OLED data acquisition, T2 and ADC mapping by SE-EPI with and without 16-time averaging were acquired as references.
Result & Discussion
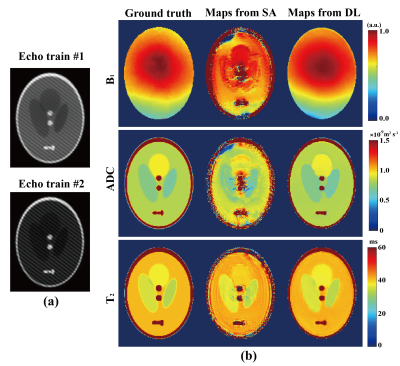
The numerical experimental results in Fig. 2 illustrate that the reconstruction based on deep learning (DL) is more robust than the one based on separation algorithm (SA). The ADC, T2, and B1 maps reconstructed from deep learning are in consistent with the corresponding references. DT2M-OLED has almost the same k-space for the first echo train as DM-OLED.3 Theoretically, separation algorithm is capable to reconstruct separated images from all six echoes and deliver parametric maps. However, 1) echo3 and echo6 for B1 estimation makes k-space more crowded. 2) The echo with diffusion weighting also has heavier T2 weighting (echo1) than the echo without diffusion weighting (echo2) in the first echo train and echo6 with diffusion weighting owns the heaviest T2 weighting, which intensifies the differences between overlapping echoes. These factors corrupt the quality of reconstructed images via separation algorithm. Therefore separation algorithm is not efficient enough for DT2M-OLED reconstruction.Fig. 3 illustrates the results of a rat brain with completely anesthetized. The ADC, B1 and T2 maps from DT2M-OLED show a good concordance to those from SE-EPI in all maps. The statistic segmentation results further verify the reliability of DT2M-OLED. The fiber orientations reflected by the ADC values are consistent with the physiological structure reported previously.4
Fig. 4 exhibits the results of a rat brain suffered from motion effect and lower signal-to-noise ratio (SNR). The T2 maps show good consistency with each other while rat was moving. No signal averaging was conducted in both sequences acquisition. The parametric maps from DT2M-OLED exhibit a bit smoother due to low SNR, but still show better contrast than SE-EPI results without signal averaging. These results indicate that DT2M-OLED is quite robust under motion environment. Self-registration between T2 and ADC maps makes DT2M-OLED competent for cooperative diagnosis via T2 and ADC mapping.
Conclusion
This study proposed an ultrafast method to deliver self-registered T2 and ADC maps in a single acquisition, which offers the possibility of bringing joint T2 and ADC imaging into clinical routine with unprecedented efficiency and motion robustness. Deep-learning based reconstruction further enhances the accuracy of parametric maps recovery. As a time-efficient multi-parametric imaging method, DT2M-OLED may facilitate multi-parametric quantitative diagnosis and dynamic quantitative MRI.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers U1805261, 11761141010, 11775184 and 82071913.References
1. Cai CB, Wang C, Zeng YQ, et al. Single-shot T2 mapping using overlapping-echo detachment planar imaging and a deep convolutional neural network. Magn. Reson. Med. 2018;80:2202-2214.
2. Zhang J, Wu J, Chen SJ, et al. Robust Single-shot T2 mapping via multiple overlapping-echo acquisition and deep neural network. IEEE Trans. Med. Imaging 2019;38:1801-1811.
3. Ma LC, Cai CB, Yang HY, et al. Motion-tolerant diffusion mapping based on single-shot overlapping-echo detachment (OLED) planar imaging. Magn. Reson. Med. 2018;80: 200-210.
4. Wu D, Xu J, Mcmahon MT, et al. In vivo high-resolution diffusion tensor imaging of the mouse brain. NeuroImage 2013;83:18-26.
Figures