0088
Diffuse axonal injury has a specific multidimensional MRI signature in traumatically injured corpus callosum1National Institute of Child Health and Human Development, Bethesda, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Synopsis
Can microscopic diffuse axonal injury lesions following trauma be seen non-invasively? We report that simultaneously integrating multiple MRI dimensions - T1, T2, and diffusion - can be targeted to image microscopic damage. Corpora Callosa derived from eight subjects that sustained TBI and three healthy control brain donors underwent post-mortem ex-vivo MRI at 7T. Multidimensional-, diffusion tensor-, and quantitative T1- and T2-MRI data were acquired and processed, along with corresponding pathohistological data. Although invisible using the conventional MRI modalities, multidimensional MRI provided images of the microscopic injuries, suggesting that it can be used for the detection of microscopic axonal injury.
Introduction
Traumatic brain injury (TBI) presents major medical, social and economic challenges world-wide and is associated with high mortality, co-morbidities, and long-term disabilities.1 Among the various pathological brain lesions produced by impact, diffuse axonal injury (DAI) is caused by mechanical damage to axons multifocally throughout the white matter (WM).2 Axonal transport is disrupted following axonal injury and pathologic accumulation of different axonal proteins causes axonal swelling, which leads to axonal degeneration.3 Amyloid precursor protein (APP) is one of those accumulated proteins, and it can be detected immunohistochemically within a few hours post injury.4 Because of that, and due to its high sensitivity and robustness, APP remains the gold-standard for the identification of axonal injury.3Conventional neuroradiological tools, such as CT and MRI, are insensitive to DAI caused by trauma. Diffusion tensor imaging (DTI) has been established as a sensitive tool for depicting axonal integrity in regions with TBI-associated lesions.5 In particular, the fractional anisotropy (FA) had stood out as being a sensitive biomarker for TBI. Unfortunately, despite DTI’s high sensitivity, conflicting findings of either decreases6-7 or increases5,8 of the FA in TBI groups compared with control subjects have been reported.
The fundamental obstacle for using MRI to detect microscopic tissue alterations is the averaging that occurs across the voxels. Voxel-averaged images can only provide macroscopic information with respect to the voxel size (typically on the order of 1 mm3), thus limited by MRI’s relatively low image resolution. Multidimensional MRI is an emerging approach that combines T1, T2, and diffusion and replaces voxel-averaged values with distributions,9,10 which allows selective isolation of specific potential abnormal components.11-13 By performing a combined post-mortem multidimensional MRI and histopathology study, we aimed to investigate T1-T2-diffusion changes linked to DAI and to define their histopathological correlates.
Methods
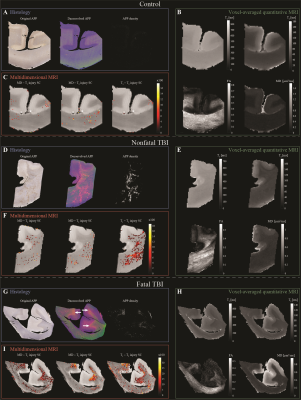
Corpora Callosa derived from eight subjects that sustained TBI, and three control brain donors underwent post-mortem ex vivo MRI at 7 T. Multidimensional-, diffusion tensor-, and quantitative T1- and T2-MRI data were acquired and processed. Following MRI acquisition, slices from the same tissue were tested for APP immunoreactivity to define DAI severity. A robust image co-registration method was applied to accurately match MRI-derived parameters and histopathology, after which 12 regions of interest per tissue block were selected based on APP density, but blind to MRI. In total, 132 regions of interest from 11 cases were included in this study. Abnormal multidimensional T1-T2, diffusion-T2, and diffusion-T1 components that are strongly associated with DAI were identified using a spectral thresholding algorithm,14 and were then used to generate axonal injury images.Results
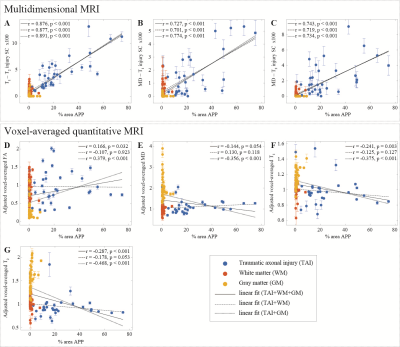
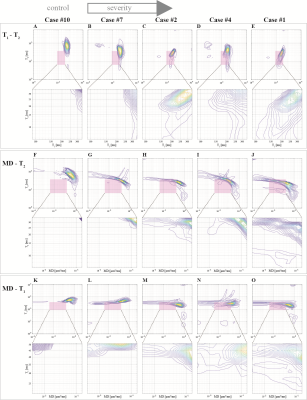
We observed a consistent and gradual change in the way in which T1, T2, and MD were distributed as the APP-based severity of the DAI was increasing. Figure 1 shows five cases with increased injury severity and their respective T1-T2 (A-E), MD-T2 (F-J), and MD-T1 (K-O) distributions. Qualitatively, the magnified regions show a gradual shortening of T1 and T2 as the severity of the injury is increased (Fig. 1, left to right).We grouped the 132 tissue regions into controls, normal-appearing WM in TBI patients, and DAI lesions in TBI patients. We further partitioned the TBI patients into mild and severe traumatic axonal injury (mTAI, sTAI) according to the APP staining-based severity. We found that compared to control white matter, mild and severe DAI lesions contained significantly larger abnormal T1-T2 component (P=0.005 and P<0.001, respectively), and significantly larger abnormal diffusion-T2 component (P=0.005 and P<0.001, respectively). Furthermore, within patients with TBI the multidimensional MRI biomarkers differentiated normal-appearing white matter from mild and severe DAI lesions, with significantly larger abnormal T1-T2 and diffusion-T2 components (P=0.003 and P<0.001, respectively for T1-T2, and P=0.022 and P<0.001, respectively for diffusion-T2). Conversely, none of the conventional quantitative MRI parameters were able to differentiate lesions and normal-appearing white matter (Fig. 3).
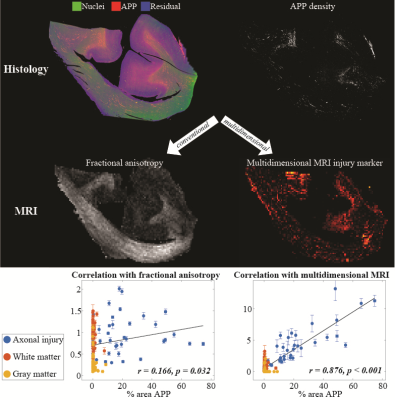
Lastly, we found that the abnormal T1-T2, diffusion-T1, and diffusion-T2 components and their axonal damage images were strongly correlated with quantitative APP staining (r = 0.876, P < 0.001; r = 0.727, P < 0.001; and r = 0.743, P < 0.001, respectively), while producing negligible intensities in gray matter and in normal-appearing white matter (Fig. 4).
Discussion
This study crucially identified potential imaging biomarkers of DAI pathology from the joint analysis of multidimensional MRI and histopathological data in the Corpus Callosum. The ability to selectively extract a specific T1-T2-MD spectral range, or spectral signature, and provide its corresponding image, allows multidimensional MRI to achieve good separation between subjects and microscopic lesion and non-lesion regions. We found that the multidimensional MRI axonal injury images are significantly and strongly correlated with histological evidence of DAI.We believe that the improved sensitivity of these novel injury biomarkers towards DAI is advancing the neuroimaging field closer towards noninvasive quantitative “histology”. This neuroimaging tool provides an injury-only image that may help clinicians and clinical investigators to detect and visualize microscopic DAI lesions in the brain. As evidence that indicates DAI is a likely pathological substrate for concussion is mounting,15 the importance of developing a means to detect it clinically becomes clear.
Acknowledgements
We thank the subjects’ families that consented for brain donations for the better understanding of TBI consequences. The authors thank Dr. Jessica Ettedgui for fruitful discussions, and Dr. Thaddeus Haight for insights into the statistical analysis. We also thank Mrs. Patricia Lee, Mrs. Nichelle Gray and Mr. Paul Gegbeh for their valuable technical work. We are grateful to Mrs. Stacey Gentile, Mrs. Deona Cooper and Mr. Harold Kramer Anderson for their administrative assistance. We thank the TRACK-TBI Investigators.References
- Faul M, Coronado V. Epidemiology of traumatic brain injury. In: Grafman J, Salazar AM, editor(s). Handbook of Clinical Neurology: Traumatic Brain Injury, Part I. Oxford, UK: Elsevier; 2015. p. 3–13.
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol 1982; 12: 557–563.
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246: 35–43.
- Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. β-Amyloid precursor protein (βAPP) as a marker for axonal injury after head injury. Neurosci Lett 1993; 160: 139–144.
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion Tensor Imaging Detects Clinically Important Axonal Damage after Mild Traumatic Brain Injury: A Pilot Study. J Neurotrauma 2007; 24: 1447–1459.
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 2002; 23: 794–802.
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011; 134: 449–463.
- Borich M, Makan N, Boyd L, Virji-Babul N. Combining Whole-Brain Voxel-Wise Analysis with In Vivo Tractography of Diffusion Behavior after Sports-Related Concussion in Adolescents: A Preliminary Report. J Neurotrauma 2013; 30: 1243–1249.
- Benjamini D, Basser P. Use of marginal distributions constrained optimization (MADCO) for accelerated 2D MRI relaxometry and diffusometry. J Magn Reson 2016; 271: 40–45.
- Benjamini D, Basser PJ. Multidimensional correlation MRI. NMR Biomed 2020: e4226.
- Benjamini D, Basser PJ. Magnetic resonance microdynamic imaging reveals distinct tissue microenvironments. Neuroimage 2017; 163: 183–196.
- Kim D, Doyle EK, Wisnowski JL, Kim JH, Haldar JP. Diffusion-relaxation correlation spectroscopic imaging: A multidimensional approach for probing microstructure. Magn Reson Med 2017; 78: 2236–2249.
- Benjamini D, Hutchinson EB, Komlosh ME, Comrie CJ, Schwerin SC, Zhang G, et al. Direct and specific assessment of axonal injury and spinal cord microenvironments using diffusion correlation imaging. Neuroimage 2020; 221: 117195.
- Benjamini D, Iacono D, Komlosh ME, Perl DP, Brody DL, Basser PJ. Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain 2020; doi: 10.1093/brain/awaa447 .
- Smith DH, Stewart W. ‘Concussion’ is not a true diagnosis. Nat Rev Neurol 2020; 16: 457–458.
Figures
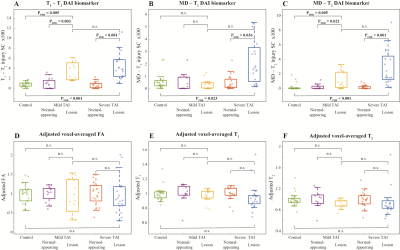
Between-group comparisons of voxel-averaged and multidimensional biomarkers and histopathological measures. Box plots showing between-group differences among control CC, normal-appearing WM in mTAI CC, mTAI lesions, normal-appearing WM in sTAI CC, and sTAI lesions. All parameters are averaged across their corresponding spatial ROIs. Note that all adjusted voxel-averaged parameters were obtained after dividing them by the mean for that parameter across all WM within the subject, to correct for possible between-subject differences arising from postmortem effects.