0087
High temporospatial resolution MR imaging of neuronal activity in vivo1Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 2Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 3Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Brain and Cognitive Engineering, Korea University, Seoul, Korea, Republic of
Synopsis
Advanced non-invasive functional imaging methods have been widely used, but with certain limitations in either temporal or spatial information. There has long been a demand for a noninvasive imaging method capable of capturing neuronal activity with high temporal and spatial resolution. Here, we demonstrate a novel imaging method (called DIANA-fMRI) for directly detecting neuronal activity with high temporal (=5ms) and spatial (=0.22mm) resolution. DIANA-fMRI was capable of capturing sensory responses in mice at 9.4T with statistically significant signal changes (~0.1-0.2%). Temporally sequential DIANA responses were also confirmed along the thalamocortical pathway, together with further validation by electrophysiological experiments.
Introduction
There has been a long demand for a non-invasive imaging method that is capable of capturing neuronal activity with a convergence of high temporal and spatial resolution. While phantom studies1,2 showed the possibility of neuronal current imaging, the in vivo direct detection of neuronal activity was considered skeptical because even some in vivo studies that reported success in this work3–5 has never been replicated. Last year, we reported very preliminary in vivo result of our new imaging method (dubbed DIANA-fMRI) for direct imaging of neuronal activity (DIANA) with high temporal resolution (=5ms), where DIANA-fMRI was performed for functional brain imaging of single mice in vivo using electrical stimulation on a whisker pad at 9.4T. Here, we updated the progress of DIANA-fMRI by presenting more validating results based on group analysis in comparison with the unstimulated controls and postmortem mice. In addition, temporally sequential DIANA responses were also confirmed along the thalamocortical pathway, together with further validation by electrophysiological experiments.Methods
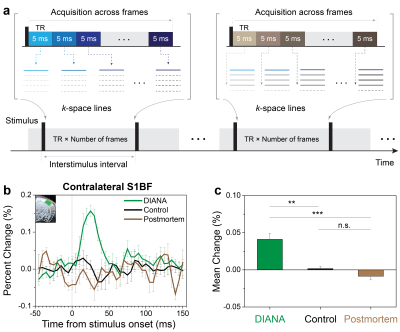
DIANA-fMRI acquisition: The key idea of our study is to increase the temporal resolution as similar as possible to the time scale of neuronal activity on the order of milliseconds, in order to effectively capture the transient effects of neuronal activity. To implement this idea, we combined the line-scan method6 and gradient-echo imaging, which acquires a single line of k-space in a time series during each interstimulus period7–9 (Fig. 1a).DIANA-fMRI experiment: In vivo mouse brain imaging was performed at 9.4T (Bruker, BioSpec 94/30). Adult C57BL/6 male mice were used under the approval of IACUC at Sungkyunkwan University and Korea University. DIANA-fMRI was conducted to acquire a time series of 40 single coronal slices with 5ms temporal and 0.22mm (in-plane) spatial resolution (Fig. 2a). Scan parameters were: TR/TE, 5/2ms; FA, 4o; FOV, 16×12mm2; matrix size, 72×54; slice thickness, 1mm; scan time, 10.8 s/trial; 40 trials/each animal. Single-pulse current stimulation (strength, 0.5mA; duration, 0.5ms) was repeatedly applied to the left whisker pad every interstimulus period of 200ms (=40×5ms) which consisted of 50ms pre-stimulation and 150ms post-stimulation. For comparison, DIANA-fMRI was also performed using unstimulated controls and postmortem mice.
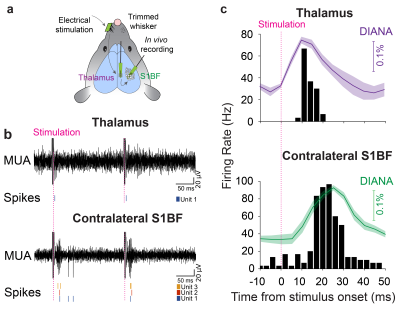
Electrophysiological recordings: For further validation, in vivo electrophysiological recordings of responsive single-unit activities were conducted simultaneously in both thalamus and contralateral barrel cortex (S1BF), with the same stimulation scheme used for DIANA-fMRI (Fig. 3a).
Data analysis: All data analyses were performed with the home-built Matlab codes (Mathworks). For preprocessing of DIANA-fMRI data, temporal smoothing (15ms kernel) and linear detrending were applied to the time series. For activation mapping, spatial smoothing was applied first with a median filter (5-voxels kernel), and each image in the post-stimulation period was compared voxel-wise with the baseline (defined as the mean of pre-stimulation signals) using paired t-test. Positive t-value voxels were displayed and overlaid on the original DIANA images with the statistical criteria of p<0.05 and cluster size > 5 voxels. For electrophysiological data, and the responsive single unit were sorted using KiloSort10 with a threshold of 4.5 S.D. after filtering multiunit activity signals (0.3–5kHz).
Results
Figure 1b shows the DIANA time series in contralateral S1BF with a response peak at ~25ms after stimulation (n=5), whereas there was no signal change in unstimulated controls (n=5) and postmortem mice (n=4). Figure 1c shows a statistical comparison between the mean signal changes of DIANA response, unstimulated controls, and postmortem mice.Figure 2b shows the sequential DIANA responses in the order of thalamus and S1BF with statistically significant signal changes (n=10, paired t-test; thalamus, 0.157±0.011%, p<0.0001; contralateral S1BF, 0.161±0.009%, p<0.0001). There was a response delay of ~10–15ms between thalamus and S1BF. Figure 2c shows the time series of t-value maps in a representative mouse, exhibiting the temporospatial distribution of brain activation in thalamus and contralateral S1BF.
Figure 3b shows the MUA (top) and responsive single-unit spikes (bottom) acquired by electrophysiological recordings. Figure 3c reveals that the spike firing rate of the responsive single units reached a peak ~10ms in thalamus and ~24ms in contralateral S1BF after stimulation, which is consistent with the DIANA responses and validates the ability of DIANA-fMRI to directly detect the neuronal activity in vivo.
Discussion and Conclusion
We successfully demonstrated the performance of DIANA-fMRI through whisker-evoked sensory responses in mice in vivo. Percent signal changes of ~0.1–0.2% were observed and, thanks to high temporal resolution (=5ms), a response delay could be resolved between thalamus (~10–15ms) and S1BF (~20–30ms) along the thalamocortical pathway, which agrees with the previous studies11–13. In terms of the signal source, we hypothesize that DIANA response is attributed to changes in T1 and T2 due to changes in membrane potential14, or partly due to cell swelling15. Further investigation is needed for elucidating the contrast mechanism of DIANA-fMRI and translating it into the human system. DIANA-fMRI is expected to open up a new horizon in functional brain imaging with high temporospatial resolution.Acknowledgements
This work was mainly supported by NRF-2019M3C7A1031993 and partly supported by IBS-R015-D1 at the beginning.References
1. Bodurka, J. & Bandettini, P. A. Toward direct mapping of neuronal activity: MRI detection of ultraweak, transient magnetic field changes. Magn. Reson. Med. 47, 1052–1058 (2002).
2. Bodurka, J. et al. Current-Induced Magnetic Resonance Phase Imaging. J. Magn. Reson. 137, 265–271 (1999).
3. Xiong, J., Fox, P. T. & Gao, J.-H. Directly mapping magnetic field effects of neuronal activity by magnetic resonance imaging. Hum. Brain Mapp. 20, 41–49 (2003).
4. Chow, L. S., Cook, G. G., Whitby, E. & Paley, M. N. J. Investigating direct detection of axon firing in the adult human optic nerve using MRI. NeuroImage 30, 835–846 (2006).
5. Chow, L. S., Dagens, A., Fu, Y., Cook, G. G. & Paley, M. N. J. Comparison of BOLD and direct-MR neuronal detection (DND) in the human visual cortex at 3T. Magn. Reson. Med. 60, 1147–1154 (2008).
6. Yu, X., Qian, C., Chen, D., Dodd, S. J. & Koretsky, A. P. Deciphering laminar-specific neural inputs with line-scanning fMRI. Nat. Methods 11, 55–58 (2014).
7. Lee, J., Lee, S.-K. & Park, J.-Y. A novel method for direct detection and spatial mapping of neuronal activity. in Proceedings of Scientific Meeting of the International Society for Magnetic Resonance in Medicine vol. 26 0703 (2018).
8. Toi, P. T., Lee, H., Lee, J., Lee, S.-K. & Park, J.-Y. Progress towards direct in-vivo detection and mapping of neuronal activity. in Proceedings of Scientific Meeting of the International Society for Magnetic Resonance in Medicine vol. 27 2991 (2019).
9. Toi, P. T. & Park, J.-Y. In vivo direct MR imaging of mouse whisker sensory responses. in Proceedings of Scientific Meeting of the International Society for Magnetic Resonance in Medicine vol. 28 3821 (2020).
10. Pachitariu, M., Steinmetz, N. A., Kadir, S. N., Carandini, M. & Harris, K. D. Fast and accurate spike sorting of high-channel count probes with KiloSort. in Advances in Neural Information Processing Systems 29 (eds. Lee, D. D., Sugiyama, M., Luxburg, U. V., Guyon, I. & Garnett, R.) 4448–4456 (Curran Associates, Inc., 2016).
11. Temereanca, S., Brown, E. N. & Simons, D. J. Rapid Changes in Thalamic Firing Synchrony during Repetitive Whisker Stimulation. J. Neurosci. 28, 11153–11164 (2008).
12. Jang, H. J. et al. Distinct roles of parvalbumin and somatostatin interneurons in gating the synchronization of spike times in the neocortex. Sci. Adv. 6, eaay5333 (2020).
13. Leong, A. T. L. et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc. Natl. Acad. Sci. 113, E8306–E8315 (2016).
14. Min, K. et al. A change in membrane potential induces measurable changes in relaxation times. in Proceedings of Scientific Meeting of the International Society for Magnetic Resonance in Medicine vol. 28 0173 (2020).
15. Janz, C., Speck, O. & Hennig, J. Time-resolved measurements of brain activation after a short visual stimulus: new results on the physiological mechanisms of the cortical response. NMR Biomed. 10, 222–229 (1997).
Figures