0077
CEST-MRI guided sequential drug delivery using injectable hydrogel for local treatment in the brain
Xiongqi Han1, Jianpan Huang1, and Kannie Wai Yan Chan1,2,3
1Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 2Biomedical Engineering, Shenzhen Research Institute, City University of Hong Kong, Shenzhen, China, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
1Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 2Biomedical Engineering, Shenzhen Research Institute, City University of Hong Kong, Shenzhen, China, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Glioblastoma (GBM) is the most malignant brain tumor with notorious heterogeneous, infiltrative and chemoresistance. Here, we developed a hydrogel-based drug delivery system for sequential and sustainable local drug delivery with rheological properties that favor applications to the brain. We designed methotrexate (MTX) to release prior to gemcitabine (Gem). Using CEST, the multicomponent of hydrogel matrix could be detected at 3 T, including the contrast of loaded drugs (5.4%) at 2.2-2.4 ppm and liposomal hydrogel matrix at -3.6 ppm in vitro. Furthermore, it showed combined cytotoxicity on U87 cells, demonstrating its potential on CEST MRI guided local treatment.
Mainbody of the abstract
Introduction: GBM is the most common and aggressive type of brain tumor with highly heterogeneous, infiltrative and chemoresistance, resulting in poor prognosis and tumor recurrence.1 Hydrogel-based local drug delivery systems have been reported for GBM treatment as it increased the drug availability at the lesion and avoided the systemic side effects.2 Moreover, hydrogels are suitable for multi-drugs delivery, which is vital for addressing drug resistance to achieve combined treatment.3 Gem is a nucleoside-like prodrug, which undergoes sequential phosphorylation in cells, inducing the inhibition of DNA replication and GBM cell apoptosis.4-5 Its low efficacy resulted from short half-life could be well solved by loading into liposomes.6 MTX is a folic acid analog, triggering cells death through the inhibition of purine and pyrimidine synthesis.7-8 Moreover, it can enhance immunosuppressive capability in GBM treatment.9 Besides, previous studies have demonstrated CEST could be applied for monitoring of hydrogel matrix and hydrogel-based drug delivery.10-16 Here, we designed a combined GBM local treatment by loading MTX and Gem-loaded liposomes (Gem-lipo) into alginate hydrogel, which demonstrated unique sequential and sustainable release, and combined anti-U87 efficacy. Moreover, drugs and liposomes generated natural CEST contrast at 2.4 and -3.6 ppm respectively at 3T, enabling CEST monitoring of drug release in vitro longitudinally and facilitating the image-guided local treatment. Methods: Gem-lipo was prepared by typical thin-film hydration method with the composition of DPPC, cholesterol and DSPE-PEG2000 at a molar ratio of 1:1:0.026.17 The resulting lipid (25 mg/mL) film was hydrated with Gem solution (20 mg/mL, pH 7.0) under 55 oC for 1 h, then sonicated, extruded and filtrated by sephadex column. MTX (5 mg/mL) and alginate powder (10 mg/mL) were then added in. The resulting mixture was mixed with calcium D-gluconate solution (4.32 wt%) at a volume ratio of 10:1 to form MGLH hydrogel. The control GLH, MLH and LH hydrogels were prepared accordingly, but without loading MTX, Gem and both drugs, respectively. The rheological, drug release and CEST measurements were the same as our previous study.10 Results: Both drugs displayed the inherent CEST signal (Figure 1) correlated with drug concentrations. Gem generated contrast at 2.2 ppm (20.0% at 50 mM), while MTX at 2.6 and 1.4 ppm (more prominent, 13.6% at 50 mM). Gem-lipo was monodispersed with the size about 300 nm (Table 1). Moreover, it produced CEST contrast (Figure 2) at 2.2 (5.8±1.2%) and -3.6 (5.1±1.1%) ppm under the optimized B1 power (1.0 μT), respectively generated from intraliposomal Gem and lipid bilayer of liposomes.18-19 The optimized MGLH hydrogel (Figure 3) exhibited CEST contrast at 2.4 ppm (5.4±0.2%) for loaded drugs and -3.6 ppm (5.0±0.5%) for the liposomal hydrogel matrix. The contrast at 2.4 ppm continuously decreased from 5.4% to 2.1% over 7 days while negligible variations at -3.6 ppm. This 61% decrease (2.4 ppm) could reflect the release of loaded drugs. Furthermore, MGLH hydrogel showed outstanding sequential release, with 65.5±4.2% of MTX and 42.9±0.9% of Gem released at 24 h. Its storage modulus (G’, 333.2±26.9 Pa) matches well to the typical brain tissue (0.1-1.0 kPa).20-21 Compared with the hydrogel only (LH) and single drug-loaded liposomal hydrogel (GLH and MLH), MGLH hydrogel treated cells showed the lowest viability (52.8 ± 7.1%). Furthermore, MGLH has negligible swelling after immersing in PBS for 24 hours. Discussion: To provide an image-guided sequential drug delivery system for local treatment in the brain, here we developed dual-drugs loaded MGLH hydrogel with decent CEST contrasts at 2.4 and -3.6 ppm. Due to the overlapping of individual drug contrast, MGLH hydrogel showed averaged drug contrast at 2.4 ppm. MGLH exhibited prominent sequential drug release with cumulative release of 65.5% MTX and 42.9% Gem at 24 h. As MTX was loaded in the hydrogel, it quickly released to surroundings. While Gem was loaded into the liposomes before hydrogel formation, which provided additional physical barrier to slow down its release. This sequential release could help minimize the toxicity towards normal cells.22,23 Since liposomes were mainly retained in hydrogel matrix (Figure 3B), hence the contrast at -3.6 ppm from liposomes could be used to monitor hydrogel and its degradation.18-19 This result was consistent to our previous study, where liposomes were mainly retained in the hydrogel after brain injection.10 Hence, CEST enabled the monitoring of drug release and hydrogel simultaneously in MGLH hydrogels. Moreover, MGLH showed the highest cytotoxicity on U87 cells (Figure 3C) because of the combined drugs’ efficacy. Concomitant with non-invasive imaging guidance, negligible swelling and brain compatible modulus, MGLH could be a promising approach for GBM local treatment. Conclusion: In summary, we developed a hydrogel-based drug delivery platform for GBM treatment, where Gem released from liposomes was slower than MTX released from hydrogel. MGLH generated CEST contrast at both positive (2.4 ppm) and negative (-3.6 ppm) region, respectively from chemotherapeutics and the rNOE of lipids, making it feasible for monitoring drug release and hydrogel in vitro. Most of all, MGLH presented the highest cytotoxicity on U87 cells than the mono-drug loaded formulations, indicating the promising of this image-guided and combined treatment on GBM.Acknowledgements
Funding Support: Research Grants Council: 11102218; City University of Hong Kong: 7005210, 9680247, 9667198 and 6000660; National Natural Science Foundation of China: 81871409References
Reference (1) Lesniak, M. S.; Brem, H. Targeted therapy for brain tumours. Nat. Rev. Drug Discov. 2004, 3 (6), 499-508. (2) Bastiancich, C.; Danhier, P.; Preat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J Control Release 2016, 243, 29-42. (3) Bastiancich, C.; Bozzato, E.; Luyten, U.; Danhier, F.; Bastiat, G.; Preat, V. Drug combination using an injectable nanomedicine hydrogel for glioblastoma treatment. Int J Pharm 2019, 559, 220-227. (4) Bastiancich, C.; Vanvarenberg, K.; Ucakar, B.; Pitorre, M.; Bastiat, G.; Lagarce, F.; Preat, V.; Danhier, F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release 2016, 225, 283-293. (5) Bastiancich, C.; Bastiat, G.; Lagarce, F. Gemcitabine and glioblastoma: challenges and current perspectives. Drug Discov Today 2018, 23 (2), 416-423. (6) Federico, C.; Morittu, V. M.; Britti, D.; Trapasso, E.; Cosco, D. Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int J Nanomedicine 2012, 7, 5423-36. (7) Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl Mater Interfaces 2018, 10 (15), 12341-12350. (8) Mujokoro, B.; Madani, F.; Esnaashari, S. S.; Khosravani, M.; Adabi, M. Combination and Co-delivery of Methotrexate and Curcumin: Preparation and In Vitro Cytotoxic Investigation on Glioma Cells. Journal of Pharmaceutical Innovation 2019. (9) Figueiró, F.; de Oliveira, C. P.; Bergamin, L. S.; Rockenbach, L.; Mendes, F. B.; Jandrey, E. H. F.; Moritz, C. E. J.; Pettenuzzo, L. F.; Sévigny, J.; Guterres, S. S. Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic signalling 2016, 12 (2), 303-312. (10) Han, X.; Huang, J.; To, A. K. W.; Lai, J. H. C.; Xiao, P.; Wu, E. X.; Xu, J.; Chan, K. W. Y. CEST MRI detectable liposomal hydrogels for multiparametric monitoring in the brain at 3T. Theranostics 2020, 10 (5), 2215-2228. (11) Dorsey, S. M.; Haris, M.; Singh, A.; Witschey, W. R. T.; Rodell, C. B.; Kogan, F.; Reddy, R.; Burdick, J. A. Visualization of Injectable Hydrogels Using Chemical Exchange Saturation Transfer MRI. Acs Biomaterials Science & Engineering 2015, 1 (4), 227-237. (12) Lock, L. L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; Cui, H. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11 (1), 797-805. (13) Liang, Y. J.; Bar-Shir, A.; Song, X. L.; Gilad, A. A.; Walczak, P.; Bulte, J. W. M. Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials 2015, 42, 144-150. (14) Zhu, W.; Chu, C.; Kuddannaya, S.; Yuan, Y.; Walczak, P.; Singh, A.; Song, X.; Bulte, J. W. M. In Vivo Imaging of Composite Hydrogel Scaffold Degradation Using CEST MRI and Two‐Color NIR Imaging. Adv. Funct. Mater. 2019, 29 (36), 1903753-1903762. (15) Jin, T.; Nicholls, F. J.; Crum, W. R.; Ghuman, H.; Badylak, S. F.; Modo, M. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials 2017, 113, 176-190. (16) Shazeeb, M. S.; Corazzini, R.; Konowicz, P. A.; Fogle, R.; Bangari, D. S.; Johnson, J.; Ying, X. Y.; Dhal, P. K. Assessment of in vivo degradation profiles of hyaluronic acid hydrogels using temporal evolution of chemical exchange saturation transfer (CEST) MRI. Biomaterials 2018, 178, 326-338. (17) Pattni, B. S.; Chupin, V. V.; Torchilin, V. P. New Developments in Liposomal Drug Delivery. Chem Rev 2015, 115 (19), 10938-66. (18) van Zijl, P. C.; Yadav, N. N. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011, 65 (4), 927-948. (19) van Zijl, P. C. M.; Lam, W. W.; Xu, J.; Knutsson, L.; Stanisz, G. J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 2018, 168, 222-241. (20) Rowland, M. J.; Parkins, C. C.; McAbee, J. H.; Kolb, A. K.; Hein, R.; Loh, X. J.; Watts, C.; Scherman, O. A. An adherent tissue-inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials 2018, 179, 199-208. (21) Engler, A. J.; Sen, S.; Sweeney, H. L.; Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677-689. (22) Xing, Y.; Chen, H.; Li, S.; Guo, X. In vitro and in vivo investigation of a novel two-phase delivery system of 2-methoxyestradiol liposomes hydrogel. Journal of liposome research 2014, 24 (1), 10-16. (23) Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N. T.; Valibeik, A.; Karimi, M.; Hamblin, M. R. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34.Figures
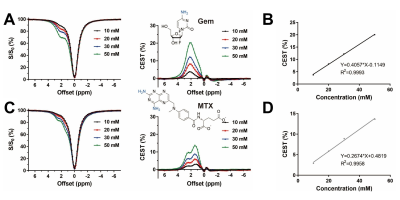
Figure 1. CEST contrast of Gem and MTX (n=3).
The representative Z-spectra and corresponding CEST percentage of
Gem (A) and MTX (C) at various concentration under 1.4 μT. The
linear fitting curve between their respective CEST magnitudes and concentration,
Gem at 2.2 ppm (C) and MTX at 1.4 ppm (D).
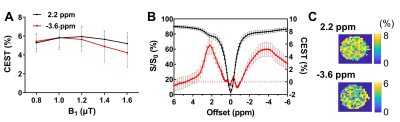
Figure 2. CEST contrast of Gem-lipo (n=4). The CEST contrast amplitudes at 2.2 ppm and
-3.6 ppm under various B1 power (A).
The Z-spectra and corresponding CEST contrast (B), and maps (C)
under 1.0 μT.
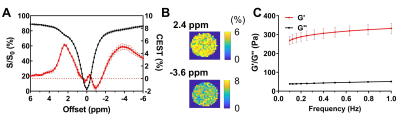
Figure 3. CEST contrast and rheology of
MGLH (n=3). The Z-spectra and corresponding CEST contrast (A), and maps
(B) under 1.0 μT. The frequency sweep
measurements of MGLH hydrogel (C).
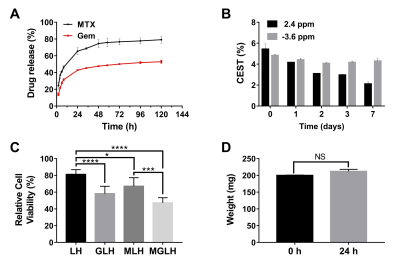
Figure 4. Drug release, longitudinally
CEST monitoring, cytotoxicities and swelling of the MGLH (n=3). (A) MTX
and Gem release profiles by UV-vis measurements. (B) the longitudinal CEST tracking of drug release and hydrogel
matrix using contrast at 2.4 ppm (drugs) and -3.6 ppm (hydrogel matrix) on day
0, 1, 2, 3, and 7, respectively. (C) the relative cell viabilities treated with
these hydrogels, using PBS treated group (100%) for normalization. (D)
the swelling tests by gravimetric measurement. P > 0.05, NS; P < 0.05,*; P<0.01,**; P<0.001,***; P<0.0001,****.