0070
One-heartbeat cardiac CINE imaging via jointly regularized non-rigid motion corrected reconstruction1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Technical University of Munich, Munich, Germany, 3Department of Computing, Imperial College London, London, United Kingdom
Synopsis
Motion-resolved reconstructions are standard for cardiac CINE imaging by splitting data into multiple cardiac phases across several heartbeats. Consequently, the reconstruction of each phase is highly ill-posed, since only a subset of the data are used to reconstruct it. In this work a novel motion corrected framework is developed for highly accelerated cardiac CINE imaging. Each cardiac phase is reconstructed with a motion corrected reconstruction and jointly regularized with every other motion corrected cardiac phase via a non-rigidly aligned patch-based denoiser. This approach leads to a substantial improvement in image quality, enabling highly accelerated CINE images from a single heartbeat.
INTRODUCTION:
Cardiac CINE imaging is commonly obtained via retrospective cardiac gating, by binning the acquired k-space data into different cardiac phases (i.e. bins). Motion resolved Compressed Sensing reconstructions have enabled considerable acceleration factors for this application primarily by exploiting the redundant information along time.1,2,3 Motion corrected reconstructions4,5,6,7 can make use of all the acquired data when the motion is known, which has higher theoretical performance. Here we extend the general motion correction formulation4 to include soft-weighting and propose a novel regularization term based on non-rigidly aligned patch-based reconstruction (PROST).8 By reconstructing each cardiac phase as a motion corrected image the effective undersampling factor is substantially reduced, leading to improved image quality and enabling single-slice CINE imaging from a single heartbeat. The proposed single heartbeat Motion Corrected CINE (MC-CINE) reconstruction was tested in five healthy subjects and compared to conventional iterative SENSE (itSENSE)9 and motion resolved XD-GRASP reconstructions.1METHODS:
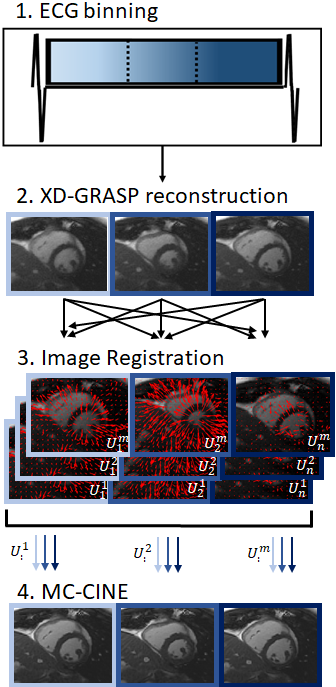
The proposed framework can be divided in four steps: 1) ECG-binning; 2) auxiliary XD-GRASP reconstruction; 3) motion estimation via image registration; and 4) motion corrected reconstruction for each cardiac frame (Fig.1). A preliminary motion resolved reconstruction using XD-GRASP (temporal total variation) is performed to produce an initial CINE reconstruction; these images are used to estimate the non-rigid cardiac motion between all cardiac phases via free-form deformation image registration.10,11 Once the motion fields between all cardiac phases are known the proposed MC-CINE is applied to reconstruct motion corrected images for each cardiac phase:$$ L(x)= argmin_{x} ||W_n(Σ_nA_nFCM_nx-k)||_2^2 + \lambda Σ_b ||T_b||_* , s.t. T_b=Q_b((M_n)^Hx)$$
where $$$W_n$$$ are soft-weights for cardiac phase n, $$$A_n$$$ is the corresponding sampling trajectory, $$$F$$$ is the Fourier transform, $$$C$$$ are coil sensitivities, $$$M_n=[U_n^1,...,U_n^m]^T$$$ are the motion fields from each cardiac phase m towards every cardiac phase n, $$$x=[x^1,...,x^m]^T$$$ are the motion corrected CINE images for each cardiac phase m, $$$k$$$ is the acquired k-space data and $$$Q_b$$$ assembles a 3D PROST tensor from non-rigidly aligned cardiac phases for a local patch around pixel b.
EXPERIMENTS:
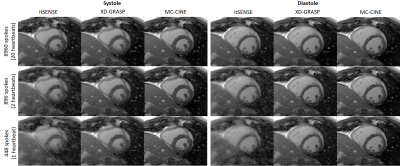
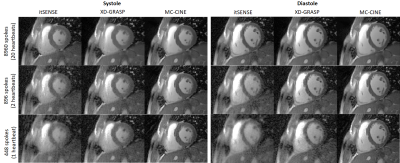
Five healthy subjects were scanned on a 1.5T scanner (Philips Ingenia). Imaging parameters included field of view (FOV) = 256x256 mm2; 8 mm slice thickness; resolution = 2x2 mm2; TE/TR = 1.16/2.3 ms; radial tiny golden angle; flip angle 60º; 8960 radial spokes acquired; nominal scan time ~20s; breath-hold acquisition. Data were reconstructed to produce 20 cardiac phases using itSENSE, XD-GRASP and the proposed MC-CINE (n=m=20) with three different retrospective acceleration factors of 1x (8960 spokes), 10x (896 spokes) and 20x (448 spokes), corresponding to effective acquisition times of ~20s, 2s and 1s, respectively.RESULTS:
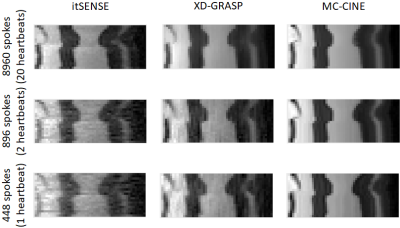
Cardiac CINE frames from two representative subjects are shown in Fig.2 and Fig.3 for itSENSE, XD-GRASP and MC-CINE at multiple acceleration factors. Residual streaking and blurring artefacts are observed with itSENSE, particularly with 896 and 448 spokes. These artefacts are reduced with XD-GRASP, but some blurring and residual incoherent aliasing can still be observed, particularly with 448 spokes. MC-CINE achieves the sharpest images with minimal artefacts, such that images with 448 spokes (1s acquisition) have comparable quality to XD-GRASP with 8960 spokes (20s acquisition). In Fig.4 a CINE animation shows normal cardiac contraction for all methods; however, considerably more artefacts are observed for itSENSE (column 1) and XD-GRASP (column 2), particularly with increasing acceleration. Consistent image quality is obtained with MC-CINE (column 3) even with 1s worth of data (row 3). A 1D temporal profile for a representative subject is shown in Fig.5 where all the dynamics of cardiac motion are correctly captured by all methods, with higher blurring and residual noise/incoherent aliasing observed for XD-GRASP and itSENSE. Again, MC-CINE with 448 spokes (1s acquisition) presents comparable quality to itSENSE and XD-GRASP with 8960 spokes (20s acquisition).CONCLUSION:
A novel approach based on Motion Corrected CINE (MC-CINE) reconstruction regularized by a non-rigidly aligned patch-based denoiser is proposed and compared with conventional itSENSE and motion resolved XD-GRASP. MC-CINE reconstructions from 1s CINE data acquisition present comparable quality to itSENSE and XD-GRASP reconstructions from 20s data acquisition. The proposed approach could enable higher resolution for CINE, real-time exercise CINE (i.e. cardiac and respiratory motion corrected) and/or whole-heart multi-slice coverage from a single breath-hold which will be investigated in future work.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC (EP/P001009, EP/P032311/1, EP/P007619/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magnetic resonance in medicine. 2016 Feb;75(2):775-88.
2. Lingala S, Hu Y, Dibella E, Jacob M (2011) Accelerated dynamic MRI exploiting sparsity and low-rank structure: K-t SLR. IEEE Trans Med Imaging 30(5):1042–1054. https://doi.org/10.1109/TMI.2010.2100850
3. Royuela-del Val J, Cordero-Grande L, Simmross-Wattenberg F, Martín-Fernández M, Alberola-López C (2017) Jacobian weighted temporal total variation for motion compensated compressed sensing reconstruction of dynamic MRI. Magn Reson Med 77(3):1208–1215. https://doi.org/10.1002/mrm.26198
4. Batchelor PG, Atkinson D, Irarrazaval P, Hill DL, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005 Nov;54(5):1273-80.
5. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free‐breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
6. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion‐corrected 3D whole‐heart coronary vessel wall imaging. Magnetic resonance in medicine. 2017 May;77(5):1894-908.
7. Xue H, Kellman P, LaRocca G, Arai AE, Hansen MS. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. Journal of Cardiovascular Magnetic Resonance. 2013 Dec 1;15(1):102.
8. Bustin A, Ginami G, Cruz G, Correia T, Ismail TF, Rashid I, Neji R, Botnar RM, Prieto C. Five‐minute whole‐heart coronary MRA with sub‐millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D‐PROST reconstruction. Magnetic resonance in medicine. 2019 Jan;81(1):102-15.
9. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k‐space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Oct;46(4):638-51.
10. Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE transactions on medical imaging. 1999 Aug;18(8):712-21.
11. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, Ourselin S. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine. 2010 Jun 1;98(3):278-84.
Figures