0061
Multimodal magnetic resonance elastography and optical imaging of breast cancer
Bin Deng1,2,3, Mansi Saksena2,3, Steven Jay Isakoff3,4, Ralph Sinkus5, Samuel Patz3,6, and Stefan Alexandru Carp1,2,3
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Cancer Center, Massachusetts General Hospital, Boston, MA, United States, 5Laboratory for Vascular Translational Science (LVTS), Institut National de la Santé et de la Recherche Médicale (INSERM), Paris, France, 6Department of Radiology, Brigham and Women’s Hospital, Boston, MA, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Cancer Center, Massachusetts General Hospital, Boston, MA, United States, 5Laboratory for Vascular Translational Science (LVTS), Institut National de la Santé et de la Recherche Médicale (INSERM), Paris, France, 6Department of Radiology, Brigham and Women’s Hospital, Boston, MA, United States
Synopsis
Breast cancers are complex, evolving systems characterized by profound spatial and temporal heterogeneity in their biological nature. A multimodal multiparametric approach is needed to synergistically use imaging methods with different biophysical basis to simultaneously quantify multiple aspects of tumor physiology. We built a custom breast coil to allow multimodal near-infrared diffuse optical tomography and MR elastography imaging of human breast. Results of a three-inclusion dual-contrast phantom showed clear contrasts in reconstructed mechanical and optical properties as expected. Data on a breast cancer patient showed collocated hemoglobin and stiffness contrast at the tumor location.
Introduction
Imaging plays a fundamental role in the management of breast cancer, contributing to a steady decrease of breast cancer mortality in the past decades. Currently, routine clinical breast imaging modalities are predominantly morphology-based and could benefit from the addition of innovative functional imaging methods that provide key features of cancerous tissue linked to tumor malignancy, aggressiveness and metastatic potential. Multimodal, multiparametric breast cancer imaging is one such approach to synergistically use imaging methods with different biophysical basis to simultaneously quantify multiple aspects of tumor physiology. In this work, we present a multimodal magnetic resonance elastography (MRE) and near-infrared diffuse optical tomography (NIR-DOT) method for breast cancer imaging. Tumors are known to have increased stiffness due to increased collagen deposition in extracellular matrix, high cellularity and higher interstitial pressure1. MRE, a novel imaging technique that measures viscoelastic tissue properties using motion-sensitizing gradients to image the propagation of low-frequency waves2, can offer insights on these biomechanical factors that play vital roles in tumor progression and treatment response. On the other hand, NIR-DOT directly quantifies key tissue components i.e., oxy- and deoxy-hemoglobin (HbO, HbR) to offer biomarkers3,4 such as total hemoglobin concentration (HbT=HbO+HbR) and mean tissue hemoglobin oxygenation to allow noninvasive assessment of tumor oxygen metabolism, perfusion and proliferation. Used together with standard clinical breast MRI exams, this multimodal approach can provide complementary tumor anatomical, functional and biomechanical features to assist the assessment of breast tumors.Methods
We have developed an 8-channel unilateral breast coil with optical fibers for NIR-DOT arranged on each side of a set of sagittal parallel plates and an MRE actuator placed in between (Fig. 1). A TechEn CW6 system equipped with 32 continuous-wave sources (split evenly between 690nm and 830nm wavelengths) and 32 detector channels is used to acquire DOT measurements5 at 1Hz for 60s concurrently with T1 scans. The passive MRE actuator is driven through a fiberglass shaft by an electromagnetic MRE transducer consisting of several turns of copper wire and is powered by a signal generator coupled to an audio power amplifier that plays back 30-100Hz sine waves when triggered by the MRE pulse sequence6 operated on a 3T Siemens MRI scanner. The AC current established in the loop causes vibrations along B0 that induce shear motion to the breast of a human subject or a tissue phantom through the padded passive actuator. Sequence details: FLASH readout, 25 degrees flip angle, TE=7.38ms, TR=165ms, FOV 192x192mm, 1.5-mm isotropic resolution, 15 slices, 4 wave phases, 4 MEG-encoding directions (1:46 TA each), 30mT/m MEG amplitude, GRAPPA acceleration factor of 2 with 24 pre-scan calibration lines. To validate the multimodal MRE/DOT approach, a three-inclusion (20-mm diameter each) dual-contrast tissue phantom was made using gelatin and agar doped with 20% Intralipid and India ink. Concentrations of agar and India ink were varied to create mechanical and absorption contrasts of the tissue phantom, respectively. A 33-y.o. breast cancer patient diagnosed with high-grade HER2+ invasive ductal carcinoma measured 4.5×2.6×2.6cm was enrolled under a protocol approved by the IRB at MGH, and multimodal MRE/DOT data were collected after written informed consent was obtained. MRE data were acquired using 100Hz vibration frequency for phantom and 50Hz for the human subject.Results and Discussion
Fig. 2 and Table 1 present the multimodal imaging results for a tissue phantom with three inclusions. The weight/volume percentage concentrations (w/v%) of agar in the gelatin phantom was targeted to yield 2x, 3x and 3x mechanical contrast, respectively, to the background. As expected, shear wavelengths seen in the phase image (Fig. 2b) are clearly longer in inclusions than in the background. Also note that shear waves can been seen propagating throughout the entire 12-cm diameter phantom, a similar dimension to the size of a human breast. Reconstructed shear moduli (Fig. 2c) also show clear contrasts in corresponding inclusion locations. However, contrast ratios are lower than target, which could partially be attributed to variations in conditions, such as mixing temperatures and curing time7, when making the individual inclusions. The reference absorption coefficients of the inclusions measured by a standard multi-wavelength frequency-domain near-infrared spectrometer resulted in a 2x, 3x and 4x optical absorption contrasts in three inclusions to the background. The image of absorption coefficient at 690nm ($$$\mu_{a}690$$$) reconstructed from the DOT data acquired using the multimodal method successfully recovered the contrasts for the two inclusions fully covered in the optical FOV. Finally, in images of a breast cancer patients shown in Fig 3, though similarly enhanced as seen in the conventional post-contrast image (Fig. 3a), the internal heterogeneity was captured by the images of total hemoglobin concentration (HbT, Fig. 3b) and shear modulus ($$$\mid{G^{*}}\mid$$$ , Fig. 3c). The elevated HbT seen in the upper right region of the tumor is likely due to angiogenesis typically seen on DOT images in malignant tumors. As a result, the pressure in interstitial fluid is likely to increase and subsequently causes stiffing of extracellular matrix in the peripheries of the tumor.Conclusion
We have demonstrated simultaneous near-infrared DOT and MRE to simultaneously investigate the biomechanical and vascular features of breast lesions. Results in a breast cancer patient show the promise of this multimodal approach in imaging the heterogeneity within a tumor.Acknowledgements
This project is supported by NIH grants K01EB027726, R01CA187595 and R01EB028664, and the European Union’s Horizon 2020 grant 668039.References
- Jain RK, “Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers,” J Clin Oncol. 2013;31(17):2205-18.
- Sinkus R, Siegmann K, Xydeas T, Tanter M Claussen C and Fink M, “MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography,” Magn Reson Med. 2007; 58(6):1135-44.
- Fang Q, Selb J, Carp SA, et al., “Combined optical and X-ray tomosynthesis breast imaging,” Radiology. 2011; 258(1):89-97.
- Sajjadi AY, Isakoff SJ, Deng B, et al., “Normalization of compression-induced hemodynamics in patients responding to neoadjuvant chemotherapy monitored by dynamic tomographic optical breast imaging (DTOBI),” Biomed Opt Express. 2017;8(2):555-69.
- Zimmermann BB, Deng B, Singh B, et al., “Multimodal breast cancer imaging using coregistered dynamic diffuse optical tomography and digital breast tomosynthesis,” J Biomed Opt. 2017; 22(4):046008.
- Garteiser P, Sahebjavaher RS, Ter Beek LC, et al., “Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence,” NMR Biomed. 2013; 26(10):1326-35.
- Madsen EL, Hobson MA, Shi H, Varghese T and Frank GR, “Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms,” Phys Med Biol. 2005;50(23):5597-618.
Figures
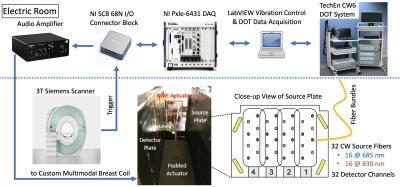
Fig. 1: Schematic of multimodal MRE/DOT acquisition system. Four phased-array elements are placed on each side of a pair of parallel plates with optical fibers arranged in between. VE fiducials are placed on four corners for registering simultaneously collected DOT and MRI images. The MRE transducer assembly consists of a padded passive actuator placed in between the two parallel plates connected to an active electromagnetic transducer located outside the FOV. Triggered amplified current drives the assembly to vibrate along B0 in synchronization with the MRE pulse sequence.

Fig. 2: Multimodal MRE/DOT imaging results of a dual-contrast tissue phantom. (a) T1 image showing three 20-mm diameter inclusions marked in circles. (b) MRE phase image obtained using 100Hz vibration showing wave propagation within the entire 12-cm diameter phantom with longer wavelengths inside inclusions. (c) Reconstructed shear modulus map and (d) absorption coefficient map shows clear contrasts in expected inclusion locations. Inclusion 3 was out of the optical coverage marked by the vertical dotted line in subplot (d), resulting in failure to recover its optical contrast.
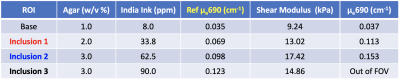
Table 1: Recipes, reference optical properties (represented as the absorption coefficients μa at 690nm near-infrared wavelength), and reconstructed mechanical and optical properties from multimodal MRE/DOT measurements of a three-inclusion tissue phantom made of gelatin, agar, 20% Intralipid, India ink and water.

Fig. 3: Multimodal images of a 33-y.o. breast cancer patient diagnosed of high-grade HER2+ invasive ductal carcinoma measured 4.5×2.6×2.6 cm. (a) T1-weighted fat saturated (FS) post-contrast MRI. (b) DOT image of total hemoglobin concentration (HbT) overlaid with simultaneously acquired T1 non-FS MRI. (c) Image of shear modulus measured by MRE. Red line – tumor marking. White dotted line – MRE actuator contact.