0057
Multiparametric MRI signatures of immune response in patients with HER2+ breast cancer treated with trastuzumab1University of Washington, Seattle, WA, United States, 2Vanderbilt University, Nashville, TN, United States, 3University of Illinois, Chicago, IL, United States
Synopsis
We investigated the relationship between immune infiltration and imaging metrics derived from breast MRI in patients with HER2+ breast cancer treated with trastuzumab. Fourteen patients with localized HER2+ breast cancer were imaged with diffusion-weighted and dynamic contrast-enhanced (DCE-) MRI prior to and ~2 weeks after a run-in dose of trastuzumab. Pre-treatment ADC and change in DCE-MRI peak percent enhancement were both significantly associated with immune response as characterized by change in level of tumor infiltrating lymphocytes.
INTRODUCTION
Trastuzumab, a monoclonal antibody targeting the HER2/neu receptor, is a standard-of-care therapy for HER2+ breast cancer that has dramatically improved overall survival for HER2+ patients. Although a targeted therapy, response to trastuzumab is variable across patients, with <50% achieving pathological complete response when treated with trastuzumab-augmented chemotherapy in the neoadjuvant setting.1,2 Trastuzumab has the potential to invoke an immune response, and clinical studies have shown that increased tumor immune infiltration is associated with improved trastuzumab response.3 Early indicators of immune response may be valuable in the treatment of HER2+ breast cancer. Diffusion-weighted (DW-) and dynamic contrast-enhanced (DCE-) MRI report on tumor cellularity and microvascularity, respectively— biological features that may reflect immune infiltration in the tumor microenvironment. Patients typically receive chemotherapy in combination with trastuzumab treatment, making it difficult to evaluate trastuzumab-specific effects in the clinical setting. Here, we evaluated patients prior to and after a single ‘run-in’ dose of trastuzumab therapy to optimally characterize immune response rather than chemo-induced cytotoxic effects. To determine whether breast MRI is indicative of trastuzumab-induced immune response, we investigated the association between imaging metrics derived from DW- and DCE-MRI and measurements of tumor infiltrating lymphocytes (TILs) from pathology in patients with HER2+ breast cancer.METHODS
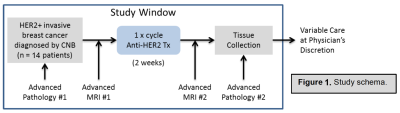
Subjects and treatment: Patients with localized HER2+ breast cancer (≥10 mm), were enrolled in this IRB approved prospective study (ClinicalTrials.gov: NCT03738553). Each patient underwent MRI prior to and approximately 2 weeks after receiving a single ‘run-in’ dose of trastuzumab. Tissue specimens were obtained via biopsy prior to treatment and via biopsy or surgical excision after treatment. Stromal TIL counts were assessed by a breast pathologist from H&E stained slides.MRI acquisition: Imaging was performed on a 3T clinical scanner (Achieva, Philips Healthcare) using a dedicated 16-channel breast coil (MammoTrak). For DCE-MRI, T1-weighted images were acquired pre- and post- injection of gadolinium-based contrast (0.1 mmol/kg-body-weight) using a fat saturated, 3D fast gradient echo sequence with post-contrast acquisitions centered at 2, 5, and 8 minutes post-injection. DW-MRI was acquired using a single shot echo-planar imaging sequence with b-values=0,100, and 800 s/mm2.
DW- and DCE-MRI processing and analysis: DCE-MRI series were first registered using CADstream software (Merge Healthcare, Chicago, IL) to correct for patient motion between pre-and post-contrast scans for each MRI exam. DCE-MRI kinetics analysis was performed using custom semi-automated software developed in ImageJ (NIH). Tumors were segmented based on at least 50% enhancement. Signal enhancement ratio (SER) and percent enhancement (PE) maps were calculated on a voxel-by-voxel basis.4 DW-MRI series were first registered using onboard Diffusion Registration software (Philips Healthcare) to correct for misalignment between different b-value acquisitions. Apparent diffusion coefficient (ADC) maps were calculated using a mono-exponential fit to DW-MRI signal intensities at b=0, 100, and 800 s/mm2. Referencing DCE-MRI for lesion localization, a tumor region-of-interest was drawn on the most representative 2D slice using a semiautomated thresholding tool on the b=800 images, which was propagated to the corresponding ADC map.5 Quantitative image metrics were calculated for each tumor at pre- and post-treatment timepoints. From DCE-MRI: peak PE (%), peak SER, functional tumor volume (FTV, cm3), and washout fraction (WF, %).6 From DW-MRI: mean ADC (x10-3 mm2/s) for most representative 2D slice.
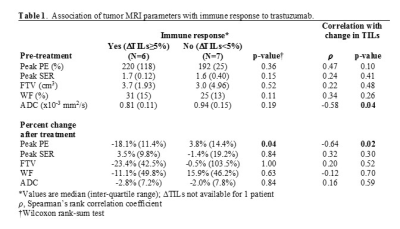
Statistical analyses: TILs and imaging features were compared between time points by Wilcoxon signed-rank test. Immune response to trastuzumab was categorized based on percent change in TILs as: response (≥5% increase in TILs) and no response (<5% change in TIL counts). Pre-treatment and change in imaging features were compared between tumors exhibiting immune response versus no response by Wilcoxon rank sum test. Spearman’s rank correlation coefficient (𝜌) was calculated to assess correlations between MRI parameters and change in TILs.
RESULTS
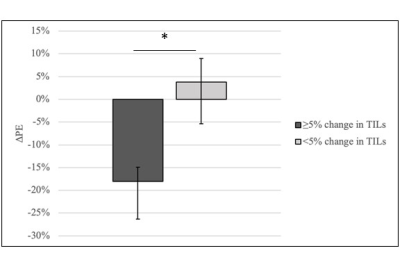
Fourteen women were enrolled and evaluated for the study (median age: 51.5, range: 37-69 years). All women underwent paired imaging and tissue sampling before and after trastuzumab therapy; post-treatment pathology was unavailable for one patient. Pre-treatment TIL counts ranged from 0% to 70% (median: 5%) and increased significantly after trastuzumab treatment (median 20%, range 0% to 90%, p=0.02). No statistically significant differences in imaging parameters were observed between pre- and post-treatment time points (p>0.05). Pre-treatment ADC and change in peak PE negatively correlated with change in TILs (𝜌= -0.58 and 𝜌= -0.64, respectively; Table 1). Moreover, tumors exhibiting an immune response showed a significantly different change in peak PE after trastuzumab treatment (median: -18.1%) compared to tumors with no immune response (median: +3.8%, p=0.04; Table 1, Figure 2). Two example cases are shown in Figure 3.DISCUSSION AND CONCLUSION
In this preliminary study, we demonstrate the potential for breast MRI to noninvasively evaluate tumor immune response to trastuzumab in patients with HER2+ breast cancer. It has been shown that trastuzumab induces vascular normalization7 and may improve tumor immune response8. Our observation of decreased peak PE in immune responders may reflect vascular normalization in terms of reduced vascular permeability and contrast extravasation. A negative correlation between ADC and change in TILs suggests that increased immune infiltration may also be associated with tumors exhibiting higher cell density. This preliminary study shows promising results warranting further investigation of imaging metrics for characterization of immune response.Acknowledgements
Supported by NIH grants P30CA015704, R01CA248192, a Roger E. Moe Fellowship (LCK), and a gift from the Safeway Foundation.References
1. Martin-Castillo, B. et al. Basal/HER2 breast carcinomas: Integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin). Cell Cycle 12, 225–245 (2013).
2. Dean-Colomb, W. & Esteva, F. J. Her2-positive breast cancer: Herceptin and beyond. European Journal of Cancer 44, 2806–2812 (2008).
3. Salgado, R. et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol 1, 448 (2015).
4. Luo, J. et al. Ductal Carcinoma in Situ: Quantitative Preoperative Breast MR Imaging Features Associated with Recurrence after Treatment. Radiology 285, 788–797 (2017).
5. Rahbar, H. et al. Diffusion-Weighted Breast Magnetic Resonance Imaging: A Semiautomated Voxel Selection Technique Improves Interreader Reproducibility of Apparent Diffusion Coefficient Measurements. J Comput Assist Tomogr 40, 428–435 (2016).
6. McDonald, E. S. et al. Mean Apparent Diffusion Coefficient Is a Sufficient Conventional Diffusion-weighted MRI Metric to Improve Breast MRI Diagnostic Performance: Results from the ECOG-ACRIN Cancer Research Group A6702 Diffusion Imaging Trial. Radiology 202465 (2020) doi:10.1148/radiol.2020202465.
7. Sorace, A. G. et al. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: preliminary results. Breast Cancer Research and Treatment 155, 273–284 (2016).
8. Song, P. N. et al. CD4 T-Cell Immune Stimulation of HER2+ Breast Cancer Cells in Response to Trastuzumab In Vitro. https://www.researchsquare.com/article/rs-45672/v1 (2020) doi:10.21203/rs.3.rs-45672/v1.
Figures