0052
Linear Time Invariant Model based Motion Compensation for Renal Function Estimation with DCE-MRI1Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Heavy breathing or large bulk motion of infants during acquisition of renal DCE-MRI causes misalignment between volumes, degrades image quality, and reduces the accuracy of estimated quantitative parameters of kidney function. We proposed a robust LTI model-based registration algorithm for motion correction, which improved the temporal stability of the concentration time curves, resulting in improved estimation of tracer kinetic model parameters such as renal filtration rate.
Introduction
Dynamic Contrast Enhanced (DCE) MRI can be used for quantitative evaluation of kidney function by fitting a tracer kinetic (TK) model onto tissue concentration curves obtained from a dynamic series of volumes and estimating the filtration rate parameter from the model. However, patient motion due to heavy breathing or large bulk motion of infant during acquisition causes misalignment between volumes before and after motion events and degrades the image quality during motion events. These effects lead to large errors in the TK model parameter estimates even when using a motion-robust dynamic radial VIBE sequence for DCE-MR imaging. The misalignments between the series of volumes are difficult to correct using standard pairwise registration of each image to a reference image due to large changes of image contrast and image geometry over time and the degradation of image quality due to motion, reducing registration accuracy. A more effective strategy is to generate motion free templates of the dynamic data using a model and to register each volume to its contrast-matched template [1]. TK model is often used to generate these templates. However, the TK models are often tissue specific, and therefore cannot be applied to every voxel in the image. They are also sensitive to motion and not robust due to need for non-linear optimization. Here instead we introduce a Linear Time Invariant (LTI) Model based motion correction (LiMo-MoCo) algorithm, where we use an LTI model to characterize the concentration time curves for all tissues. We approximate the LTI model as a sparse sum of first order LTI functions to introduce robustness to motion and artifacts.Methods
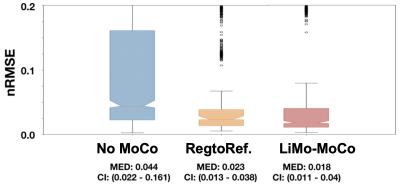
We acquired DCE-MR images from 10 infants (0 – 4 months) with hydronephrosis using an approved IRB protocol. The infants were fed, swaddled and rocked to sleep and imaged without sedation [2] using a “stack-of-stars” 3D FLASH prototype sequence with a multi-channel body-matrix coil (3T Siemens Skyra/Trio, TR/TE/FA 3.56/1.39ms/12◦, 32 coronal slices, voxel size=1.25x1.25x3mm, 1326 radial spokes acquired in 6 mins with golden angle radial ordering) and reconstructed the sequence of volumes using GRASP reconstruction [3]. We used 34 k-space spokes per slice, leading to an average temporal resolution of 3.3 s. We then applied the proposed LiMo-MoCo Algorithm for motion correction [4]. In short, the LiMo-MoCo generates a motion-free template of the series of DCE volumes to register each volume to its contrast-matched template. To generate the template, it uses a constrained atomic-norm minimization approach to fit an LTI model to each voxel. The atomic-norm regularization and the constraints produce solutions that are robust to the motion artifacts. Registration is done non-rigidly with ELASTIX. Furthermore, we used the LTI template to recover the volumes corrupted by motion. After registration, we fitted the tracer kinetic (TK) model by Sourbron et al. [5] to the concentration curves [6] and computed 100 bootstrap repetitions [7] to estimate uncertainty. We report on the goodness of fit of the TK model as the normalized root-mean-squared error (nRMSE). Statistical tests were done with Wilcoxon rank test.Results
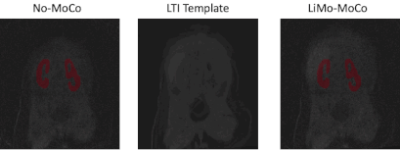
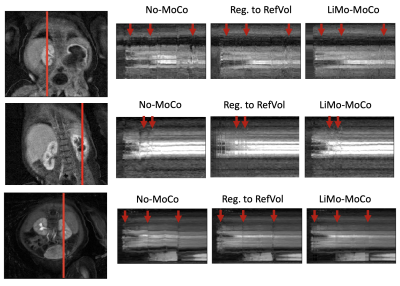
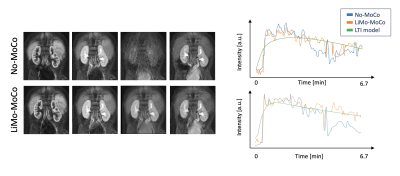
As shown in Figure 1, the templates generated by the LTI model accurately resembled the original volumes without the motion artifacts and, after applying registration, the original volumes showed no motion and corrected the corrupted volumes. As seen in Figure 2, LiMo-MoCo resulted in better alignment than the standard registration to an individual reference volume (Reg. To RefVol) and in-painted the instances with signal dropout. These effects can be clearly seen in the signal intensity curves shown in Figure 3. The original data presents large discontinuities while, after correction, these disappear. As a consequence, the TK model fitting improved. The goodness-of-fit, shown in Figure 4, was significantly improved compared to the No-MoCo and the reg. to refVol examples (p<0.05).Conclusions
Our proposed LTI model-based motion correction (LiMo-MoCo) approach for DCE-MR images of the kidneys improved the alignment of volumes when compared to no MoCo and to the standard method of registering every single volume to a reference volume. The LTI model fitting was robust to motion when compared to fitting TK models to generate templates for registration. LTI model was also used to interpolate the volumes corrupted during motion events. LiMo-MoCo method resulted in improved accuracy in estimating the tracer kinetic model parameters which are used to compute kidney function.Acknowledgements
This work was supported partially by the Boston Children's Hospital Translational Research Program Pilot Grant 2018, Society of Pediatric Radiology Multi-center Research Grant 2019, Crohn’s and Colitis Foundation of America’s (CCFA) Career Development Award and by NIDDK of the National Institutes of Health under award number R21DK123569 and by NIBIB of the National Institutes of Health under award number R21EB029627.References
[1] Buonaccorsi, G.A., et al.: Tracer kinetic model-driven registration for dynamic contrast-enhanced MRI time-series data. Magn. Reson. Med. 58(5), 1010–1019 (2007). https://doi.org/10.1002/mrm.21405
[2] S.Kurugol et. al, “Feed and Wrap Magnetic Resonance Urography Provides Anatomic and Functional Imaging in Infants Without Anesthesia,” J. Pediatr. Urol., 2019.
[3] L. Feng et al., “Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI.,” Magn. Reson. Med., vol. 72, no. 3, pp. 707–17, Sep. 2014.
[4] Coll-Font J, Afacan O, Chow JS, Lee RS, Warfield SK, Kurugol S. Modeling Dynamic Radial Contrast Enhanced MRI with Linear Time Invariant Systems for Motion Correction in Quantitative Assessment of Kidney Function. Medical Image Analysis. 2020;67:1–12. doi:10.1016/j.media.2020.101880
[5] S. P. Sourbron, H. J. Michaely, M. F. Reiser, and S. O. Schoenberg, “MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model,” Invest. Radiol., vol. 43, no. 1, pp. 40–48, 2008.
[6] Coll‐Font J, Afacan O, Chow JS, Lee RS, Stemmer A, Warfield SK, Kurugol S, Coll-Font J, Afacan O, Chow JS, et al. Bulk motion-compensated DCE-MRI for functional imaging of kidneys in newborns. Journal of Magnetic Resonance Imaging. 2019 ;52(1):207–216. https://onlinelibrary.wiley.com/doi/abs/10.1002/jmri.27021. doi:10.1002/jmri.27021
[7] R. Davidson and E. Flachaire, “The wild bootstrap, tamed at last,” J. Econom., vol. 146, no. 1, pp. 162–169, Sep. 2008.
Figures