0047
Measurement of Blood-Brain Barrier Disruption in Cats with an Inherited Neurodevelopmental Abnormality using Magnetization Transfer-ASL at 7T1Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Auburn University MRI Research Center, Auburn University, Auburn, AL, United States, 3Department of Pathobiology, Auburn University, Auburn, AL, United States, 4Scott-Ritchey Research Center, Auburn University, Auburn, AL, United States, 5Department of Anatomy, Physiology and Pharmacology, Auburn University, Auburn, AL, United States
Synopsis
The blood-brain barrier (BBB) plays a vital role in regulating nutrient transport and acts as a barrier to potentially harmful molecules. Disruption of the BBB alters normal neurodevelopment and neuronal function. Protein enriched in astrocytes 15-kDa (PEA15) is crucial in normal neurodevelopment of cats, and cats with a PEA15 loss-of-function (PEA15-/-) have structural brain abnormalities and behavioral defects. We have previously demonstrated a non-invasive MRI technique to measure BBB permeability in humans. The goal of this study is to investigate if this technique can detect differences in microvascular cerebral blood flow and BBB in PEA15-/- cats compared to PEA15+/+.
Introduction
Phosphoprotein enriched in astrocytes 15-kDa (PEA15) is an adapter protein that plays a critical role in regulating cellular morphology 1. This multifunctional protein has been shown to play a vital part in protection of neurons and various neurological diseases such as Alzheimer’s disease, Parkinson’s disease, and Schizophrenia are associated with changes in PEA15 activity and/or concentration 2. Recent discovery in our lab shows that PEA15 is crucial in neurodevelopment of cats, which is a gyrencephalic animal model 3. Cats with a loss of function PEA15 variant (PEA15-/-) have severe microcephaly and polymicrogyria. Neurodevelopment is a complex process that demands high energy supply in the form of glucose and other nutrients. Cerebral blood flow (CBF) supplies the brain with nutrients and oxygen for energy production. The blood-brain barrier (BBB) can play a very important role in regulating the transport of these nutrients and oxygen from vasculature to brain tissue. We hypothesize that the BBB is altered in PEA15-/- cats. We have previously demonstrated a non-invasive magnetization transfer (MT) based FAIR-ASL perfusion technique to quantify BBB permeability surface area product (PS) and perfusion (f) in humans at 7T 4. Brain and vascular water has different MT effects therefore it can be used to estimate the fraction of arterial blood (E) that can exchange with the tissue. The goal of this study is to adapt the technique for small animal studies and investigate how PEA15 can affect the perfusion and BBB permeability in the cat brain.Methods
All the experiments were performed with Siemens 7T Magnetom with 32 channel head coil. QUIPSS II FAIR ASL technique with additional MT pulse to saturate the macromolecules as described previously was used 4. This technique relies on the fact that tissue water and vascular water are effected differently when brain macromolecules are saturated with MT pulses 5,6 hence allowing us to quantify perfusion and water extraction fraction (E). Control (PEA15+/+) and PEA15-/-(n = 3 and total 6 slices in each group) cats were used in this study. Cats were anesthetized using dexmedetomidine (0.04 mg/kg) and ketamine (10 mg/kg) intramuscularly (IM). Once anesthetized the cats were incubated and maintained in a surgical plane of anesthesia using isoflurane. All scans were performed with the animal wrapped in towels and warm water bottles. Heart rate, respiration, oxygen saturation and body temperature were monitored by MRI-compatible equipment. MT-FAIR ASL pulse sequence was described before where MT-ON and MT-OFF acquisitions were obtained in interleaved fashion. MT pulses with duration of 16.64 ms and offset frequency 500 Hz were used to saturate the macromolecules. 8 ms hyperbolic secant adiabatic pulse was used for perfusion tagging. Other imaging parameters were: FOV=144×192 mm, matrix = 192x256, TE=2 ms, flip angle=10o, slice thickness=3 mm, bandwidth=977 Hz/pixel, perfusion tagging time = 1 sec. A total of 160 images were acquired (40 for each of Tag (MT on), Control (MT on), Tag (MT off) and Control (MT off)). A proton density reference image was also acquired to normalize the perfusion signal.Results
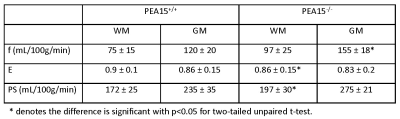
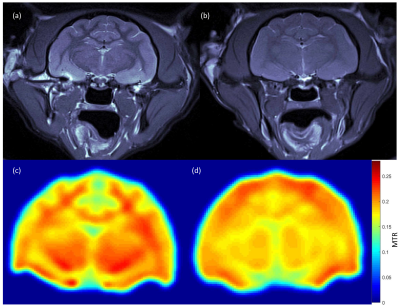
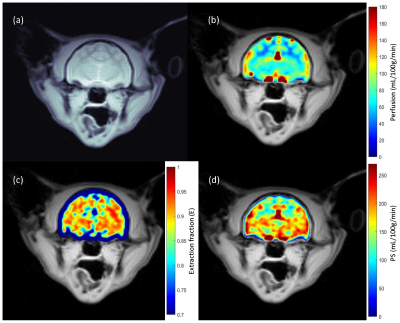
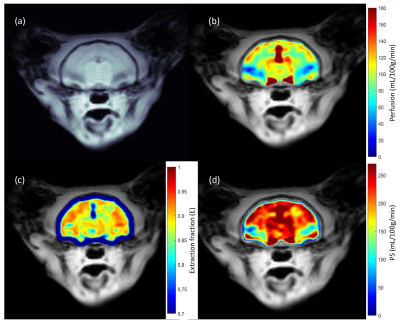
Anatomical and MTR images show severe microcephaly, polymicrogyria and lack of white matter development in PEA15-/- cats (Fig. 1). Perfusion (f) and BBB permeability (E and PS) are summarized in table 1 (mean ± sd). Average perfusion and PS were higher in PEA15-/- cats compared to PEA15+/+ showing compromised BBB (Fig. 2 and 3).Discussion
The results show that the BBB is disrupted in PEA15-/- cats. In a separate study, immunohistochemistry staining showed increased collagen in PEA15-/-, an important component of the vascular basement membrane (BM). Alteration in BMs lead to BBB dysfunction, which supports our result 7. These findings might further explain the function of PEA15 in neurodevelopment and offer a unique animal model and non-invasive technique to study brain development.Acknowledgements
No acknowledgement found.References
1. Eckert A, Bock BC, Tagscherer KE, et al. The PEA-15/PED protein protects glioblastoma cells from glucose deprivation-induced apoptosis via the ERK/MAP kinase pathway. Oncogene 2008;27(8):1155-1166.
2. Greig FH, Nixon GF. Phosphoprotein enriched in astrocytes (PEA)-15: a potential therapeutic target in multiple disease states. Pharmacol Ther 2014;143(3):265-274.
3. Graff EC, Cochran JN, Kaelin CB, et al. PEA15 loss of function and defective cerebral development in the domestic cat. bioRxiv doi: 101101/20200214949214.
4. Mahmud SZ, Denney TS, Bashir A. Measurement of Blood-Brain Barrier Permeability in Human Brain using Magnetization Transfer Effect at 7T. In Proceedings of 28th Annual Meeting of ISMRM, 2020. p 1749.
5. Balaban RS, Ceckler TL. Magnetization transfer contrast in magnetic resonance imaging. Magn Reson Q 1992;8(2):116-137.
6. Dousset V, Degreze P, Mieze S, et al. Magnetization transfer on in vitro circulating blood: Implications for time-of-flight MR angiography. Jmri-J Magn Reson Im 1995;5(6):786-788.
7. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015;7(1):a020412.
Figures