0046
Alterations in Network Connectivity Within Special Forces Military Personnel: A Combined Resting-FMRI and DTI Study1School of Medicine, Queen's University, Kingston, ON, Canada, 2Center for Neuroscience Studies, Queen's University, Kingston, ON, Canada, 3Performance Phenomics, Toronto, ON, Canada, 4National Defence Headquarters, Ottawa, ON, Canada, 5Department of Surgery, Queen’s University, Kingston, ON, Canada
Synopsis
Chronic exposure to head trauma in Special Forces personnel may provide a mechanism for changes in connectivity making-up the architectural organization of functional hubs. Here, resting-state MRI and diffusion tensor imaging are integrated to highlight interdependent differences in functional and structural connectivity of Canadian military Special Operations Forces personnel, when compared to civilian. Changes in white matter integrity of fibers directly connecting functional nodes were observed, which may explain, at least in part, changes in functional markers within networks. These findings suggest a potential structural compensatory relationship between axonal injury and neural recruitment following head trauma from high-exposure military duties.
Introduction
Military personnel who engage in duties that involve repetitive firing of large caliber weapons, or exposure to explosive devices, are exposed to a significant amount of head trauma.1 Chronic exposure to mechanical loading forces that result from such trauma may induce changes in functional and structural connectivity that make-up networks within the brain.2–5 Altogether, these findings emphasize the need to better our understanding of the effects of repeated sub-concussive injury on the relationship between structural and functional biomarkers as an avenue to improve the existing guidelines in place to balance exposure to head impacts in military personnel, while optimizing training conditions and performance during field duties.In this study, we use a multimodal approach to characterize the chronic effects of exposure to repetitive head trauma in Canadian Special Operations Forces (CANSOF) personnel, compared to control civilians with no lifetime exposure to head trauma. We hypothesized that distance-based6 changes in functional connectivity strength (FCS) would be associated with changes in white-matter (WM) integrity within the CANSOF group, as an index for the compensatory mechanisms in place within the brain to maintain architectural organization of the functional hubs that make up large-scale networks.
Methods
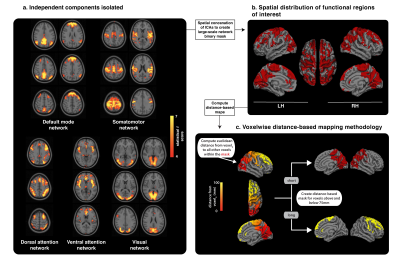
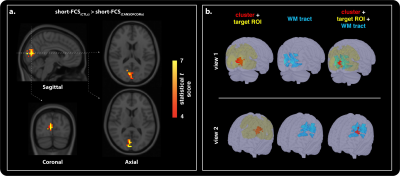
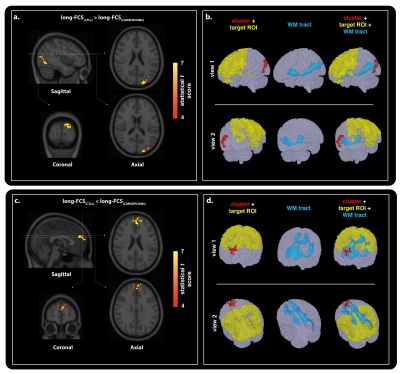
Fifteen CANSOF members and an equal age-matched cohort of civilian (CTLs) were enrolled in this cross-sectional design. Imaging was performed on a Siemens 3.0 T Magnetom Tim-Trio system using a 32-channel receiver head coil. BOLD-based rs-fMRI datasets (36 slices; matrix, 68x68; TR, 2000 ms; TE, 30 ms; voxel size, 3.5 mm isotropic; flip angle, 90°; no slide gap; field of view, 240 mm; GRAPPA imaging (acceleration factor, 2))7 were combined with DTI (60 slices; matrix, 128 mm; TR, 7800 ms; TE, 95 ms; field of view, 256 mm; slice thickness, 2 mm; voxel size, 2 mm; 30 anterior-posterior phase-encoding directions; b-value = 1000 s/mm2; GRAPPA imaging (acceleration factor, 3))7 to reconstruct the functional and structural networks using independent component analyses (Figure 1) and probabilistic tractography (Figure 2 and 3).8,9Functional and structural connectivity markers were computed based on the distance between functional seeds, in order to assess for possible group-based differences in injury susceptibility of short- (< 75mm) and long- (> 75mm) range connections within the brain (Figure 1).6 Short- and long-FCS maps were compared between the groups using a voxel-based Student t test in AFNI’s9 3dMVM.10 Results were corrected for multiple comparisons and family-wise error using a parameter-specific cluster size determined with 3dFWHMx (with spatial AutoCorrelation) and 3dClustSim,11 at a corrected P-value set to 0.05. Significant clusters were used to reconstruct the associated structural networks, as described above.
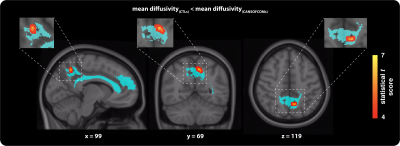
Reconstructed WM tracts were assessed for possible group differences in fractional anisotropy (FA) and mean diffusivity (MD) along the tracts using the same voxel-based statistical approach described above. All parameters showing significant cluster-based differences were extracted from each region of interest and used to perform post-hoc analyses to describe the direction of the group effect.
Results
Group comparison of functional connectivity strength revealed significant differences in short- (Figure 2) and long-range (Figure 3) cortical connections across the grey-matter, between the groups. Both hyper- and hypo-connectivity differences in functional markers were observed in the CANSOF members, compared to the CTLs (Figure 2 and 3). Significant alterations in white-matter integrity of neuronal fibers (increased mean diffusivity) directly connecting disrupted functional nodes were specific to long-range connections showing hyper-connectivity patterns only (Figure 4).Discussion
In this study, rs-fMRI and DTI were integrated to provide insight into distance-based differences in functional and structural connectivity in CANSOF personnel, in comparison to civilians. Alterations in structural integrity of fibers directly connecting functional networks may suggest potential compensatory relationship between axonal injury and neural recruitment following repetitive head trauma from high-exposure military duties.Findings from this study emphasize that multi-parametric imaging is essential for understanding the relationship between the functional and structural components that make up neurological networks and how those may be disrupted following exposure to repetitive head trauma. This study also provides an opening for further discussions about the need to combine neuroimaging, neurocognitive, biomechanical studies, along with training modification and guidelines, to ensure that training-based exposure to head trauma for military personal can be minimized, while maintaining high-level performance and operational readiness.
Acknowledgements
The authors of this study would like to thank Mr. Don Brien for his help with data collection, as well as members of the Canadian Special Operations Forces military personnel and staff for their collaboration in this study.References
1. Xue C, Ge Y, Tang B, et al. A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS One. 2015;10(3):1-21. doi:10.1371/journal.pone.0120270
2. Wilde EA, Bouix S, Tate DF, et al. Advanced Neuroimaging Applied to Veterans and Service Personnel with Traumatic Brain Injury: State of the Art and Potential Benefits. Vol 9.; 2015. doi:10.1007/s11682-015-9444-y
3. Kamimori GH, LaValle CR, Eonta SE, Carr W, Tate C, Wang KKW. Longitudinal investigation of neurotrauma serum biomarkers, behavioral characterization, and brain imaging in soldiers following repeated low-level blast exposure (New Zealand breacher study). Mil Med. Published online 2018. doi:10.1093/milmed/usx186
4. DeCicco JP, Roby PR, DeLellis SM, et al. The relationship between neurovascular coupling, vision and sensory performance, and concussion history in Special Operations Forces combat soldiers. Clin Neuropsychol. Published online 2020. doi:10.1080/13854046.2020.1783367
5. Roby PR, DeCicco JP, Chandran A, et al. Neurovascular Coupling in Special Operations Forces Combat Soldiers. Ann Biomed Eng<BR>. Published online 2020. doi:10.1007/s10439-020-02604-y
6. Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci. 2013;110(5):1929-1934. doi:10.1073/pnas.1214900110
7. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. Published online 2002. doi:10.1002/mrm.10171
8. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
9. Cox R. AFNI : Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29(3):162-173.
10. Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571-588. doi:10.1016/j.neuroimage.2014.06.027
11. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017;7(3):152-171. doi:10.1089/brain.2016.0475
Figures