0041
Improvements in neuropsychological functioning and recoveries in brain structures and functions in inactive professional fighters
Xiaowei Zhuang1,2, Lauren Bennett3, Virendra Mishra1, Zhengshi Yang1,2, Karthik Sreenivasan1,2, Aaron Ritter1, Charles Bernick1,4, and Dietmar Cordes1,2,5
1Lou Ruvo Center For Brain Health, Cleveland Clinic, Las Vegas, NV, United States, 2University of Nevada, Las Vegas, Las Vegas, NV, United States, 3Hoag Memorial Hospital Presbyterian, Newport Beach, CA, United States, 4UW Medicine, Seattle, WA, United States, 5University of Colorado Boulder, Boulder, CO, United States
1Lou Ruvo Center For Brain Health, Cleveland Clinic, Las Vegas, NV, United States, 2University of Nevada, Las Vegas, Las Vegas, NV, United States, 3Hoag Memorial Hospital Presbyterian, Newport Beach, CA, United States, 4UW Medicine, Seattle, WA, United States, 5University of Colorado Boulder, Boulder, CO, United States
Synopsis
Longitudinal changes in fighters’ cognitive performance and brain structural (cortical thickness) and functional (seeded functional connectivity) measures following their transitions to inactive fighting status were investigated and compared with fighters who remain active. A linear mixed effect model was applied for each measure. When fighters transitioned to inactive status, improvements in cognitive performances, structural thickness measures and related functional connectivity measures are evident. In contrast, in fighters who continue to compete in professional matches, neuropsychological performances and structural and functional brain measures are observed to remain largely stable or reflect subtle declines across time points.
Introduction
Repetitive head trauma common in combat sports is a risk factor for multiple neuro-degenerative disorders1,2. Previous studies have associated repetitive head injuries with both cognitive impairment and structural or functional brain damages in active professional fighters3,4. However, it remains unclear whether these noted cognitive and brain impairments will progress further, remain stable, or recover once fighters retire at a relatively young age. In this study, we investigate the longitudinal changes in fighters’ cognitive performance and brain structural and functional measures following their transitions to inactive fighting status and compare these changes with fighters who remain active.Method
Subjects. Longitudinal demographics, fighting histories, neuropsychological performance, and imaging data from 29 inactive fighters and 29 matched active fighters were obtained from the Professional Brain Health Study (PFBHS)5. At time point 1 (TP1), all 58 fighters actively participated in professional matches; at time point 2 (TP2), inactive fighters had been at least two years without a professional match while active fighters continued competing in professional matches. As listed in Table 1, active fighters were matched to inactive fighters in terms of demographics (e.g. age, sex, education, and race), number of professional fights at TP1, and time differences between TP1 and TP2. Neuropsychological measures. The CNS Vital Sign tests were used to evaluate cognitive, executive, and processing speed functions in all fighters at both TPs. Imaging data acquisition and analysis. At each TP, resting-state fMRI data were collected for all subjects on a 3T Siemens scanner (TR/TE/resolution=2.8s/28ms/2x2x4mm3, 30 slices, axial acquisition, 137 time frames). A T1-weighted structural image was also acquired with a standard MPRAGE sequence. Each T1 image was input to the FreeSurfer pipeline and a subject-specific anatomical segmentation was generated with 68 cortical regions of interest (ROI) based on Desikan-Killiany6 atlas and 12 sub-cortical regions. Cortical thickness measures for 68 cortical regions were obtained for each subject as structural measures. To compute the functional connectivity (FC) measures, the subject-specific parcellation was co-registered to fMRI native space using a 12 parameter-affine transformation. After standard preprocessing steps, average resting-state fMRI time series were obtained for 80 ROIs in each fighter. Statistical analysis. A linear mixed effect (LME) model was applied to investigate the longitudinal changes for each neuropsychological, structural, and FC measure. Fixed effects in the LME model included group (active or inactive at TP2) effect, time effect and interaction effect between group and time; additional fixed effects of age, sex, education level and race were also included in the LME model as covariates. The intercept and time varied by subject and were considered as random effects in the LME model. Regions with significant interaction effect at p<0.01 in structural thickness analysis, and their contralateral regions were used as seed regions to obtain FC measures, which were computed as the Pearson’s correlations between each seed regions and 80 ROIs.Result
As shown in Fig. 1A, at TP2, inactive fighters demonstrate improved verbal memory (VM), processing speed (PSS), psychomotor speed (PSY) performances, and a reduced reaction time (RT), as compared to TP1; whereas active fighters’ performances were largely stable, with subtle declines on measures of VM and PSY across TPs. After false discovery rate (FDR) corrections, a significant interaction effect (pFDR<0.05) remains for VM and PSY performances in the LME model (Fig. 1B). For cortical thickness measures, after FDR correction, significant time effects (pFDR<0.05) are observed in the bilateral posterior cingulate cortex (PCC) and right isthmus cingulate cortex (iCC, Fig. 2B). More interestingly, at the significance level of p<0.01, different changing trajectories across two TPs between active and inactive fighters were observed (Fig. 2A), with inactive fighters demonstrating a recovered thickness in the left insula, left rostral anterior cingulate cortex (rACC) and right rostral middle frontal cortex (rMFC), whereas active fighters show stable or slight decrease in thickness measures of these regions. These three regions, and their contralateral sides, are used as seed regions in the FC analysis. As shown in Fig. 3A and B, improved FC are observed for inactive fighters in bilateral rACC connections, whereas active fighters demonstrate stable or reduced FC values. The interaction effect of FC between right rACC and right iCC remains significant after FDR correction (Table 2). In Fig. 3D, we visualize all FC with a significant interaction effect at p<0.01, which demonstrate recoveries only in inactive fighters, but not active fighters. These are mainly frontal-occipital and frontal-cingulate connections.Discussion and Conclusion
Our findings demonstrate that when fighters transitioned to inactive status, improvements in cognitive performances, structural thickness measures and related functional connectivity measures are evident. In contrast, in fighters who continue to compete in professional matches, neuropsychological performances and structural and functional brain measures are observed to remain largely stable or reflect subtle declines across time points.Acknowledgements
This study is supported by the National Institutes of Health (grant 1R01EB014284, R01NS117547, P20GM109025 and P20AG068053), a private grant from the Peter and Angela Dal Pezzo funds, a private grant from Lynn and William Weidner, a private grant from Stacie and Chuck Matthewson and the young scientist award at Cleveland Clinic Lou Ruvo Center for Brain Health (Keep Memory Alive Foundation). The PFBHS is supported by Bellator, UFC, the August Rapone Family Foundation, Top Rank, and Haymon Boxing.References
- Bazarian, J. J., Cernak, I., Noble-Haeusslein, L., Potolicchio, S. & Temkin, N. Long-term neurologic outcomes after traumatic brain injury. J. Head Trauma Rehabil. 24, 439–451 (2009).
- Jordan, B. D. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 9, 222–230 (2013).
- Bernick, C. et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: The Professional Fighters’ Brain Health Study. Br. J. Sports Med. 49, 1007–1011 (2015).
- Mishra, V. R. et al. Understanding white matter structural connectivity differences between cognitively impaired and nonimpaired active professional fighters. Hum. Brain Mapp. hbm.24761 (2019). doi:10.1002/hbm.24761
- Bernick, C. et al. Professional fighters brain health study: Rationale and methods. Am. J. Epidemiol. 178, 280–286 (2013).
- Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Figures
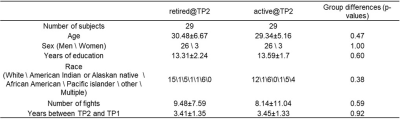
Table 1.
Demographics and fighting history of active and inactive (retired) fighters at
time point 1.
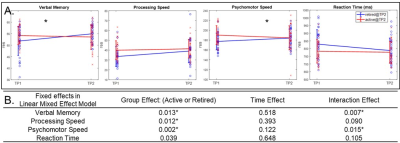
Fig.1. CNS
Vital Signs test scores. (A). Changing trajectories along time for inactive
fighters (blue) and active fighters (red). (B). Fixed effects (p-values)
in linear mixed effect model. * indicates significance after FDR correction.
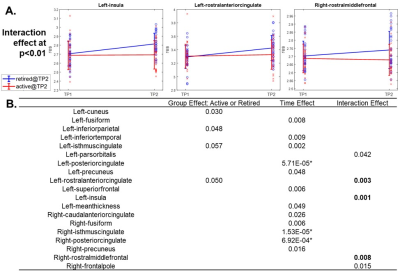
Fig 2. Structural
brain cortical thickness measures: (A). Changing trajectories along time of
regions with interaction effect at p<0.01 in the LME. These three regions,
and their counter lateral sides, were used as seed regions for later functional
connectivity analysis. (B). Fixed effects (p-values) in LME. Regions showing
effects at p<0.05 are listed. * indicates significance after FDR correction.
Regions with interaction effect at p<0.01 were used as seeds for functional
connectivity analysis.
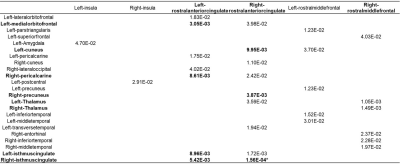
Table 2. Fixed effects (p-values) in linear mixed effect model for
seed-based functional connection measures. Regions showing interaction effect
at p<0.01 in FreeSurfer thickness analysis and their contralateral sides
were used as seeds (each column). Connections with interaction effect at
p<0.05 are shown here, with interaction effect at p<0.01 are highlighted in bold, and * indicates significance after FDR correction. Abbreviations:
rACC: rostral-anterior cingulate cortex; rMFC: rostral middle frontal cortex.

Fig. 3. Changing trajectories of seed-based functional connection
measures with interaction effect at p<0.01 with left rostral anterior
cingulate cortex (A), right rostral anterior cingulate cortex (B)
and right rostral middle frontal regions (C) as seeds. *
indicates significance after FDR correction. (D). Functional connections with
interaction effect at p<0.01 with the trends of inactive fighters
demonstrating recovery whereas active fighter showing decline or no change
across two time points.