0040
Mild Traumatic Brain Injury Predisposes to Thalamic Reticular Nucleus Impairment and Thalamocortical Dysrhythmia1Neuroscience Research Center, Taipei Medical University, Taipei, Taiwan, 2Translational Imaging Research Center, Taipei Medical University Hospital, Taipei, Taiwan, 3Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 4Graduate Institute of Biomedical Electrics and Bioinformatics, Taipei, Taiwan, 5Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 6Research Center for Artificial Intelligence in Medicine, Taipei Medical University, Taipei, Taiwan
Synopsis
This study is the first to provide strong evidence that thalamocortical dysrhythmia (TCD) is involved in mild traumatic brain injury (mTBI) and plays a crucial role in protracted symptoms. The impaired cortical–thalamic tracts and thalamic reticular nuclei are recognized as two pathomechanisms of TCD in mTBI. TCD-induced thalamocortical disinhibition, such as within-thalamus hyperconnectivity, widespread low-frequency thalamocortical coherence, and thalamo-default-mode network disinhibition, are associated with patients’ prolonged symptoms, which were consistently presented at 1- and 2-year follow-ups. Our systematic analysis strengthens understanding of TCD involvement in mTBI and provides future directions for diagnosis, prognosis, and treatment of long-lasting symptoms in mTBI.
Introduction
Neurocognitive symptoms caused by mild traumatic brain injury (mTBI), such as headache, sleep disturbances, depression, and cognitive impairment are commonly reported1. These manifestations are particularly evident during the acute stages and resolve within a few months; however, symptoms may be longer lasting in a substantial proportion of patients. A simulation model based on head-to-head collisions in professional football has been established and proved that rotational acceleration during impact can produce maximum shearing forces mainly distributed in the center of the brain2. Such forces may result in the acute disruption of cortical–thalamic connections that lasts longer than cortical dysfunction in mTBI3. Here, we hypothesized that persistent clinical symptoms after mTBI can be attributed to thalamocortical dysrhythmia (TCD), a disturbed thalamocortical oscillatory mechanism that can be investigated using diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI). We further hypothesized that the mechanism of TCD has two origins: the predisposition to injury of the thalamic reticular nucleus (TRN) and the deafferentation of cortical–thalamic tract input. This hypothesis was examined through longitudinal neuropsychological assessments and MRI evaluations at initial and 1- and 2-year follow-ups.Materials & Methods
Seventy patients with mTBI and 48 age- and gender-matched healthy volunteers consented to enter the study (Table 1). The follow-up rates at 1 and 2 years for patients with mTBI were 30% (n=21) and 12.9% (n=9), respectively. Patients with closed-head injury manifesting with loss of consciousness lasting<30 min, initial Glasgow Coma Scale score>13, and normal computed tomography were recruited. Neuropsychological assessments were performed by a clinical psychologist.MRI data were obtained using a 3T MR scanner (Siemens MAGNETOM Prisma). DTI data (EPI; TR/TE=7500/59ms; 64-direction noncoplanar diffusion gradients; voxel=0.86×0.86×3mm3), fMRI data (both resting-state and working memory (WM) task conditions; EPI; TR/TE=2000/20ms; voxel size=3×3×3.5mm3), and structural images (3D-MPRAGE; TR/TE/TI=2300/3.26/1030ms; voxel size=1×1×1mm3) were acquired. During resting-state, participants were instructed to keep their eyes closed and not engage in any particular thoughts while remaining awake and alert. During WM N-back tasks, participants were instructed to respond whenever the current stimulus matched the number that had been presented N times previously (N=1 or 2)4.
DTI data were preprocessed using FSL 5.0.10 and MRtrix 3.0.2, while fMRI data were preprocessed using SPM12. The cortical regions-of-interest were defined by a 3mm-diameter sphere centered at the WM 2-back task (de-)activation peak regions (Figure 4B), whereas the thalamic nuclei were identified based on Talairach atlas.
Results & Discussion
Significantly increased radial diffusivity (RD) and decreased fractional anisotropy (FA) at the boundaries of the bilateral thalami (Figure 1A), indicated predisposing axon and/or myelin injuries to the TRN in patients. Significantly decreased thalamocortical tract density in patients (Figure 1B) suggested the deafferentation of thalamic neurons and result in TCD5.Patients exhibited decreased thalamo-default-mode network (DMN) anticorrelation during WM task (Figure 2A), which was indicative of the dysfunction of GABAergic neurons in TRN as revealed by TCD. The thalamo-DMN anticorrelation strength during the WM task (Figure 2B,C) exhibited significant correlations with participants’ sleep quality (PSQI), postconcussion symptoms (PCSQ), arithmetic ability, and WM ability (WMI). The results suggested that less anticorrelation between the thalamus and DMN may lead to more severe clinical symptoms and poor cognitive function.
The within-thalamus hyperconnectivity observed in mTBI (Figure 3A) was positively correlated with postconcussion symptoms (PCSQ) and depression (BDI) (Figure 3B). The increased temporal coherence among the thalamic subdivisions probably resulted from the low-frequency rhythmicity produced by the conjunction of specific and nonspecific thalamic loops when TCD occurred5. This rhythmicity spreads to the cortex and promotes a resonant interaction between thalamus and cortex5. The increased coherence occurred between the thalamus and almost all cortical regions in the low-frequency band (0.01–0.08 Hz) in mTBI patients (Figure 3C). Significantly positive correlations (Figure 3D) between low-frequency thalamocortical coherence averaged across all cortical regions at low-frequency band and sleep quality (PSQI), postconcussion symptoms (PCSQ), and depression (BDI) in patients demonstrated that the low-frequency thalamocortical oscillations promoted by TCD can produce negative symptoms, such as sleep deficit, attention deficit, fatigue, and depression5,6.
Significant reduced task-induced DMN deactivation was observed in patients (Figure 4). This DMN deactivation deficit may be caused by low-frequency rhythmicity entering the thalamocortical loops spreads from the thalamus level to neighboring thalamocortical modules. This rhythmicity may further constrain cortical–cortical inhibitory interneurons and reduce lateral inhibitory drive at the cortical level7. Failure to deactivate DMN during the cognitive task may limit the ability to reallocate cognitive resources to task execution8,9. Similarly, a significant correlation (Figure 4C) between DMN deactivation strength and task accuracy was found.
The TCD-related neuroimaging biomarkers (Figure 5), including TRN demyelination, reduced thalamo-DMN anticorrelation, within-thalamus hyperconnectivity, widespread low-frequency thalamocortical coherence, and reduced DMN deactivation, as well as the TCD-related symptoms (Figure 6), such as sleep deficit (PSQI), postconcussive symptoms (PCSQ), and depression (BDI), did not diminish at the 1-year and 2-year follow-ups. These symptoms did not differ significantly between the initial and follow-up assessments, indicating that even 2 years after mTBI, the symptoms had not improved significantly.
Conclusion
Our results confirmed that mTBI may predispose patients to injuries of the TRN and thalamocortical tract, which have strong links to TCD and persistent negative effects. These findings may have critical therapeutic implications for patients with prolonged mTBI symptoms.Acknowledgements
The author(s) disclosed receipt of the following financial support for the research and publication of this article: This work was partially supported by Ministry of Science and Technology, Taiwan (MOST108-2321-B-038-008).References
1. Vanderploeg RD, Belanger HG, Curtiss GJAopm, & rehabilitation (2009) Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. 90(7):1084-1093.
2. Mendez CV, et al. (2005) Mild traumatic brain injury: neuroimaging of sports-related concussion. 17(3):297-303.
3. Moeller JJ, Tu B, & Bazil CWJAon (2011) Quantitative and qualitative analysis of ambulatory electroencephalography during mild traumatic brain injury. 68(12):1595-1598.
4. Owen AM, McMillan KM, Laird AR, & Bullmore EJHbm (2005) N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. 25(1):46-59.
5. Llinás RR, Ribary U, Jeanmonod D, Kronberg E, & Mitra PPJPotNAoS (1999) Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. 96(26):15222-15227.
6. Llinás RR & Steriade MJJon (2006) Bursting of thalamic neurons and states of vigilance. 95(6):3297-3308.
7. Sarnthein J, Morel A, Von Stein A, Jeanmonod DJT, & Systems R (2003) Thalamic theta field potentials and EEG: high thalamocortical coherence in patients with neurogenic pain, epilepsy and movement disorders. 2(3):231-238.
8. Hu Y, Chen X, Gu H, & Yang YJJoN (2013) Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. 33(47):18566-18573.
9. Singh KD & Fawcett IJN (2008) Transient and linearly graded deactivation of the human default-mode network by a visual detection task. 41(1):100-112
Figures
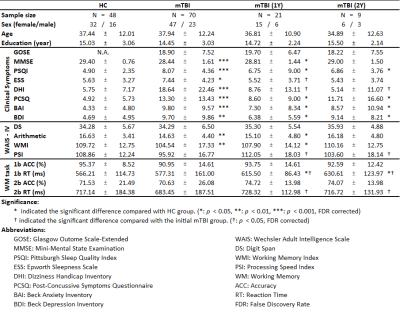
Table 1. Demographics, clinical and cognitive characteristics, and WM task performance of the enrolled individuals
Demographics or behavioral characteristics are statistically compared not only between patients with mTBI and HCs but also between the initial and follow-up data among patients with mTBI.
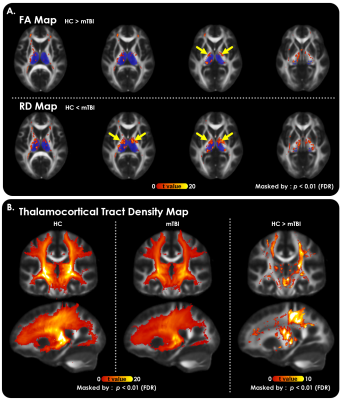
Figure 1. Structural evidence of two TCD origins in mTBI revealed by DTI.
(A) Significantly decreased FA (top row) and increased RD (bottom) at the bilateral thalamic borders (yellow arrows) were observed in patients with mTBI compared with HCs (p<0.01, FDR corrected). The ovals covered by translucent dark blue indicate the location of the bilateral thalamus. These maps were masked by the threshold of group-averaged FA > 0.2.
(B) A significant reduction in thalamocortical tract density was found in the mTBI group compared with the HC group (p<0.01, FDR corrected).
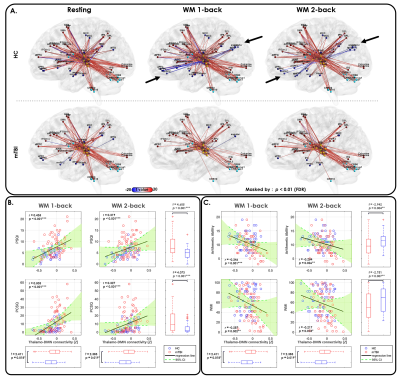
Figure 2. Thalamocortical functional connectivity changes and their clinical significance in mTBI.
(A) Patients with mTBI exhibited significantly reduced thalamo-DMN anticorrelation (black arrows) during WM 1-back and 2-back task conditions compared with the HCs.
Thalamo-DMN anticorrelation strength exhibited (B) a significant positive correlation with participants’ PSQI and PCSQ scores and (C) a significant negative correlation with participants’ arithmetic ability and WMI during the WM 1-back and 2-back task conditions.
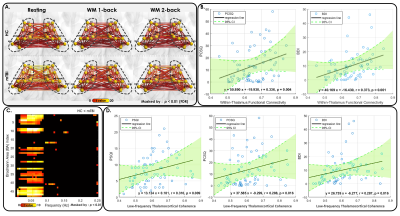
Figure 3. TCD-related functional connectivity changes and clinical symptoms in mTBI.
(A) Patients exhibited significantly increased within-thalamus connectivity compared with the HCs.
(B) The within-thalamus resting-state connectivity exhibited significantly positive correlations with PCSQ and BDI in patients.
(C) Coherence between thalamus and almost all cortical regions in the low-frequency band was increased in patients.
(D) The averaged low-frequency thalamocortical coherence exhibited significantly positive correlations with PSQI, PCSQ, and BDI in patients.
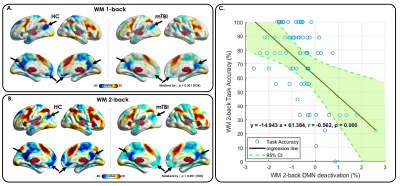
Figure 4. Reduction of WM task-induced DMN deactivation in mTBI.
(A) Compared with HCs (left), significantly reduced WM task-induced DMN deactivation was observed in patients with mTBI (right) in the WM 1-back condition.
(B) Compared with HCs (left), significantly reduced WM task-induced DMN deactivation was observed in patients with mTBI (right) in the WM 2-back condition.
(C) A significantly negative correlation was found between WM task-induced DMN deactivation strength and task accuracy during the WM 2-back condition in patients with mTBI.
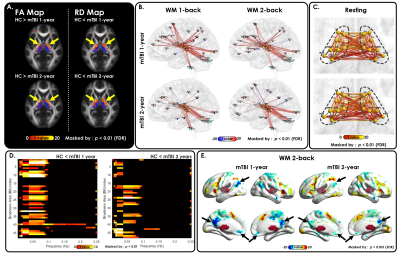
Figure 5. TCD-related neuroimaging biomarkers persisted after 1 and 2 years.
(A) Significantly increased RD and decreased FA at the bilateral thalamic borders, (B) decreased anticorrelation between the thalamus and DMN during WM 1-back and 2-back tasks, (C) hyperconnectivity among thalamic subdivisions, (D) significantly increased low-frequency thalamocortical coherence, and (E) significant reduction of task-induced DMN (black arrows) were still can be observed in patients with 1-and 2-year follow-up data.
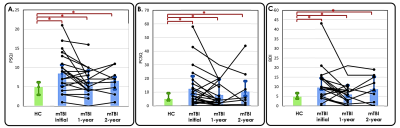
Figure 6. TCD-related clinical symptoms persisted after 1 and 2 years.
Spaghetti plot of patient-level symptom severity scores: (A) PSQI, (B) PCSQ, and (C) BDI are overlaid on the bar graphs, which indicate the average scores in each group. Significant differences in symptom severity scores were observed between HCs and patients with mTBI both initially and in follow-ups. However, no significant difference was found between the initial and follow-up symptom severity scores among patients with mTBI.