0036
Value of Liver MR Elastography for Predicting Development of Cirrhosis and Decompensation in Patients with Alcohol-associated Liver Disease1Radiology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, 2Mayo Clinic, Rochester, MN, United States, 3West China Hospital, Chengdu, China, 4the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Synopsis
Identifying those at high risk for the development of end-stage liver disease in patients with alcohol-associated liver disease (ALD) is of great clinical importance. We retrospectively investigated the role of MR elastography (MRE) in predicting cirrhosis or decompensated cirrhosis on a cohort of 182 ALD patients. Our preliminary results indicated that liver stiffness measured by 2D-MRE is a significant and independent prognostic factor for the future development of cirrhosis or decompensated cirrhosis. These results echoed the previous findings in other etiologies and reinforced the prognostic value of MRE in predicting advanced liver disease, which facilitates early intervention for ALD.
Introduction
Alcohol-associated liver disease (ALD) is one of the most prevalent chronic liver diseases worldwide 1,2. It progresses over a spectrum ranging from alcoholic fatty liver, alcoholic steatohepatitis (alcoholic hepatitis), cirrhosis, and hepatocellular carcinoma. Currently, there are no effective non-invasive techniques to identify individuals who are at high risk for disease progression. The implications of such methods would have a major effect in monitoring millions of individuals with ALD. Magnetic resonance elstography (MRE) has long been well-known as the most accurate non-invasive method to estimate liver fibrosis 3-5. Recently, more and more studies have implied that except for the assessment of liver fibrosis, MRE also has the prognostic information 6-9. A prior study has demonstrated that liver stiffness (LS) measured by MRE is a significant predictor of future development of cirrhosis in patients with nonalcoholic fatty liver disease (NAFLD) 10. However, few studies outlined the role of MRE in predicting disease progression in ALD patients. Therefore this study aims to investigate the role of MRE in predicting 1) the development of cirrhosis in initially non-cirrhotic livers, and 2) the progression from compensated to decompensated cirrhosis.Methods
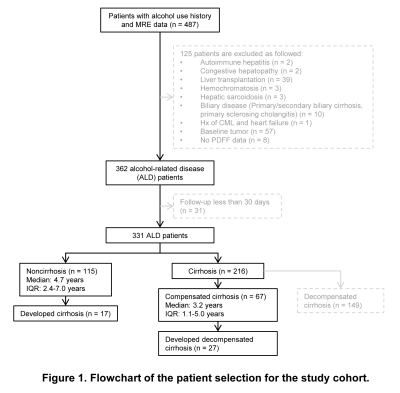
This retrospective study was approved by our Institutional Review Broad with the waiver of written informed consents. 182 patients with ALD and at least one 2D-MRE examination were included in this study (Figure 1). Patient demography, liver function tests, and MELD score within 30 days from the date of the first MRE, history of hepatic virus infection (HCV/HBV), and alcohol use history (duration of alcohol use, frequency, and last date of alcohol use) were collected from the electronic medical records. LS was retrieved from the patient’s baseline MRE examination report. An experienced radiologist measured proton density fat fraction (PDFF) by placing three regions of interest (ROIs) on the axial 2 or 6-point Dixon images at the portal vein level. Patients were followed up until they developed alcoholic cirrhosis (for patients with baseline non-cirrhosis) or decompensated alcoholic cirrhosis (for patients with baseline compensated cirrhosis). Cirrhosis was defined as follows: 1) liver biopsy within 6 months from the time of baseline MRE, or 2) LS ≥ 5 kPa, or 3) imaging features of portal hypertension or signs of decompensated cirrhosis. Decompensated cirrhosis was defined as the presence of at least one of the following: ascites, jaundice (total bilirubin > 2.5), encephalopathy, and esophageal variceal bleeding 10. Alcohol abstinence was defined as avoiding alcohol for more than three months before the first MRE examination. Univariate and multivariate Cox regression analyses were performed to identify the significant and independent prognostic (LS, PDFF, abstinence, etc.) or risk (sex, age, etc.) factors for cirrhosis or decompensated cirrhosis, respectively. A p-value of less than 0.05 was considered statistically significant.Results
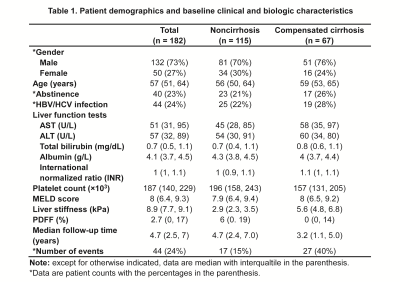
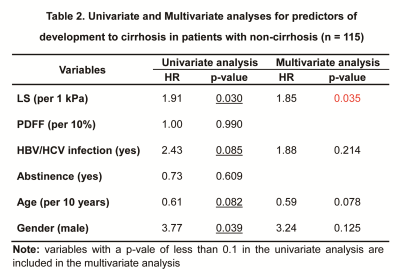
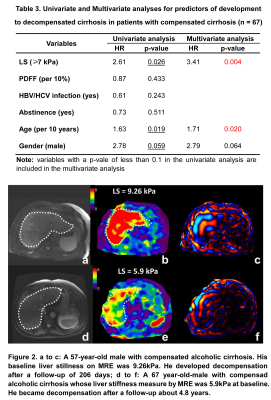
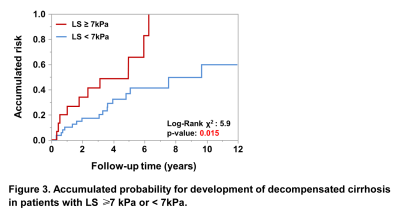
Patient demographics and baseline clinical and biologic characteristics were summarized in Table 1. A total of 182 patients with 27% (50/182) females and a mean age of 57±10 years were included in this study. 44 (24%) patients had HBV or HCV infection. 40 (22%) patients had abstinence. At the baseline, 115 (63%) patients had non-cirrhosis and 67 (37%) patients had compensated cirrhosis. 17 (15%) of 115 patients without cirrhosis developed cirrhosis after a median follow-up of 4.7 (IQR: 2.4, 7.0) years. 27 (40%) of 67 patients with compensated cirrhosis developed decompensated cirrhosis after a median follow-up of 3.2 (IQR: 1.1, 5.0). In patients with cirrhosis, Cox regression analyses revealed that after adjusting for HBV/HCV infection, age and gender, LS per 1kPa increment (HR: 1.85, p = 0.035) was the only significant and independent prognostic factor for developing cirrhosis (Table 2). In patients with compensated cirrhosis, Cox regression analyses indicated that after adjusting for age and gender, LS ≥ 7kPa (HR: 3.41, p = 0.004) was a significant and independent prognostic predictor of decompensated cirrhosis (Table 3, Figure 2).Discussion
Defining the effects of LS on the course of ALD and patient outcome, especially the development of cirrhosis or decompensated cirrhosis, would facilitate early intervention for ALD. Our results showed that the risk for developing liver cirrhosis increased by 85% for 1 kPa increment in LS, and the risk for developing decompensated in patients with LS ≥ 7kPa was about 3.5 times that of those with LS < 7kPa (Figure 3). These results echoed the previous findings 7-10 and reinforced the prognostic role of MRE in predicting advanced liver disease in addition to the evaluation of liver fibrosis. There are some limitations to our study. First, the retrospective nature of this study may cause some intrinsic biases. Second, the lack of a validation cohort to confirm our findings warrants further investigation. Third, PDFF was mostly measured using a 2-point Dixon method without correction for T1 and T2* bias (e.g. IDEAL-IQ). Further studies on the prognostic value of MRE in ALD patients are needed.Conclusion
Liver stiffness measured by MRE can predict clinical outcomes (i.e. cirrhosis and decompensated cirrhosis) in ALD patients.Acknowledgements
This study is supported by NIH: R37 EB001981 (R.L.E.); NIH: R01 EB017197 (M.Y.); NIH: UH2/3 AA026887 (V.S. & M.Y.); and DoD: W81XWH-19-1-0583-01 (M.Y.)References
1.Seitz HK, Bataller R, Cortez-Pinto H et al. Alcoholic liver disease. Nat Rev Dis Primers 2018; 4: 16
2.Avila MA, Dufour JF, Gerbes AL et al. Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut 2020; 69: 764-780
3.Xiao G, Zhu S, Xiao X et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017; 66: 1486-1501
4.Singh S, Venkatesh SK, Wang Z et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015; 13: 440-451 e446
5.Imajo K, Kessoku T, Honda Y et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016; 150: 626-637 e627
6.Ichikawa S, Motosugi U, Enomoto N et al. Magnetic resonance elastography can predict development of hepatocellular carcinoma with longitudinally acquired two-point data. Eur Radiol 2019; 29: 1013-1021
7.Han MAT, Vipani A, Noureddin N et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver Int 2020; 40: 2242-2251
8.Lee DH, Lee JM, Chang W et al. Prognostic Role of Liver Stiffness Measurements Using Magnetic Resonance Elastography in Patients with Compensated Chronic Liver Disease. Eur Radiol 2018; 28: 3513-3521
9.Singh S, Fujii LL, Murad MH et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 1573-1584 e1571-1572; quiz e1588-1579
10.Gidener T, Ahmed OT, Larson JJ et al. Liver Stiffness by Magnetic Resonance Elastography Predicts Future Cirrhosis, Decompensation and Death in NAFLD. Clin Gastroenterol Hepatol 2020, DOI: 10.1016/j.cgh.2020.09.044