0035
Tumor stiffness and stiffness change using 3D MR elastography are markers of tumor lymphocyte infiltration and immunotherapy response in HCC.1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Regeneron Pharmaceuticals Inc., Tarrytown, NY, United States
Synopsis
In this prospective study we correlated tumor stiffness measured in hepatocellular carcinoma with density of tumor infiltrating lymphocytes and assessed the changes in tumor stiffness in patients undergoing neoadjuvant immunotherapy prior to liver resection. We found that tumor stiffness strongly correlates with grade of tumor infiltrating lymphocytes, and that changes in tumor stiffness correlate with histopathologic response.
Introduction
Immune checkpoint inhibitors present new possibilities in cancer treatment (1) with encouraging results for hepatocellular carcinoma (HCC) (2). The tumor microenvironment plays a vital role in therapy response, with previous reports suggesting higher density of tumor infiltrating lymphocytes (TILs) resulted in improved immunotherapy outcomes (3). Traditional radiological methods for assessing HCC treatment response may not be best suited to immunotherapy as tumor size may not change following therapy. This has prompted the publication of immunotherapy specific response criteria known as immune response evaluation criteria in solid tumors or iRECIST (4). However, iRECIST is not applicable in the neoadjuvant setting as a second follow up exam is required to differentiate tumor progression from pseudoprogression. The objectives of our study were: 1) assess the relationship between 3D MRE tumor stiffness (TS) and density of TILs, 2) correlate stiffness measurements with tumor response in a cohort of patients undergoing neoadjuvant immunotherapy prior to liver resection.Methods
In this prospective IRB approved study, written informed consent was obtained in 20 patients who underwent MR imaging including 3D MRE at baseline and following completion of immunotherapy treatment (baseline-post treatment imaging interval 30±8 days) on a 1.5T system (Aera, Siemens). We report initial results in 14 patients (mean age 64±12y, 10 men) with HCC scheduled to undergo neoadjuvant anti PD-1 immunotherapy (cemiplimab) prior to liver resection (post treatment imaging-resection interval 4±2 days). 3D MRE was performed using a prototype SE-EPI sequence with 32 axial slices acquired at 60Hz vibration frequency. Regions of interest were prescribed in lesions, peritumoral liver parenchyma and in liver parenchyma away from tumors to measure tumor stiffness (TS) and liver stiffness. We also calculated TS change (Δ) as (post-treatment – pre-treatment TS)/pre-treatment TS. Tumor size pre- and post-immunotherapy and tumor size change was assessed by a radiologist using post-contrast images. Resected samples were assessed by a pathologist who measured degree of necrosis and assessed the grade of tumor infiltrating lymphocytes (TIL: none, localized foci, diffuse presence and abundant). Tumors with ≥70% necrosis were considered positive responders. Stiffness changes following therapy were assessed using Wilcoxon signed-rank tests. Differences between independent groups were tested for significance using Mann Whitney U tests. Association between MRE measurements and pathologic measurements was assessed using Spearman correlations.Results
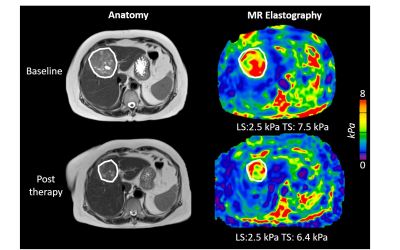
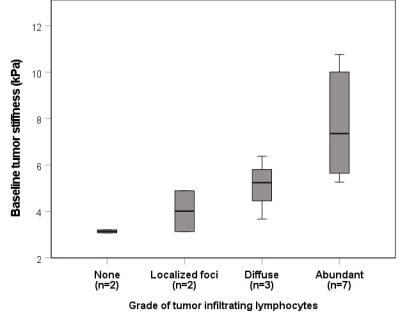
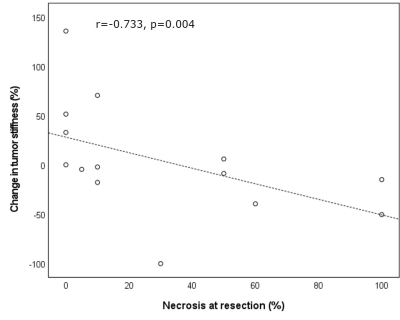
Example pre- and post-immunotherapy images are seen in Figure 1. Mean baseline tumor size was 6.2±3.8cm. 9/14 tumors had 5% or greater necrosis based on the resected liver sample, with 2 tumors achieving ≥70% necrosis (both 100% necrotic). TILs were present in 12/14 tumors at histopathology, with both tumors achieving ≥70% necrosis containing abundant TILs. MRE acquisition was unsuccessful in one post-therapy scan. Baseline TS was significantly higher in patients with abundant TILs (7.73±2.24 vs 4.23±1.28 kPa, p=0.005), and significantly correlated with grade of tumor infiltrating lymphocytes (r=0.863, p<0.001, Figure 2). Over the whole cohort there was no significant change in liver, peritumoral liver and TS post-immunotherapy (p>0.695). Tumor size was also unchanged (p=0.265). ΔTS was significantly correlated with percentage necrosis at resection (r=-0.733, p=0.004, Figure 3) while tumor size change was not (r=-0.374, p=0.188). ΔTS was significantly different between patients with ≥70% necrosis compared to patients with <70% necrosis, with patients with increased necrosis showing a reduction in TS following immunotherapy (-26.4±47.8% vs 34.8±18.1 %, p=0.014).Discussion
Initial results from this study suggest that TS correlates with density of TILs, and changes in TS reflect tumor response after neoadjuvant anti PD-1 immunotherapy. The only previous study assessing immunotherapy response with MRE also noted no overall change in TS post immunotherapy (5). In that study tumor necrosis was not determined and so no comparison of ΔTS and necrosis was reported. Previous studies assessing tumor response to locoregional (6) and antivascular (7) therapy have noted a reduction in tumor stiffness associated with tumor necrosis. Necrosis is associated with reduced cellularity and cell membrane permeability (8) which may cause a reduction in stiffness owing to increased liquid fraction. In contrast, we found that tumors which did not achieve 70% showed an increase in tumor stiffness. This may be due to increased inflammation at the tumor site.We found that stiffer tumors at baseline were more likely to contain higher densities of TILs at resection, which are linked to positive outcomes in immunotherapy (9). This finding is also supported by the study of Qayyum et al (5). The mechanism through which increased TS is associated with increased TIL density is unclear. Elevated extracellular matrix deposition may cause increased TS but is considered a barrier to immune cell infiltration (10). Other mechanisms, such as the beneficial effect of cytokines on TIL accumulation and activity in HCC (11) may also play a role.
Conclusion
Our initial results from a cohort of patients with HCC indicate that baseline TS measured with 3D MR elastography correlates with density of tumor infiltrating lymphocytes at resection following neoadjuvant immunotherapy. ΔTS was also found to correlate with degree of tumor necrosis at resection.Acknowledgements
This work was funded by Regeneron Pharmaceuticals Inc.References
1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252-264.
2. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492-2502.
3. Plesca I, Tunger A, Muller L, et al. Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front Immunol 2020;11:364.
4. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18(3):e143-e152.
5. Qayyum A, Hwang KP, Stafford J, et al. Immunotherapy response evaluation with magnetic resonance elastography (MRE) in advanced HCC. J Immunother Cancer 2019;7(1):329.
6. Gordic S, Ayache JB, Kennedy P, et al. Value of tumor stiffness measured with MR elastography for assessment of response of hepatocellular carcinoma to locoregional therapy. Abdom Radiol 2017:1-10.
7. Li J, Jamin Y, Boult JK, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer 2014;110(7):1727-1732.
8. Ross BD, Moffat BA, Lawrence TS, et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther 2003;2(6):581-587.
9. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol 2020.
10. Cohen IJ, Blasberg R. Impact of the Tumor Microenvironment on Tumor-Infiltrating Lymphocytes: Focus on Breast Cancer. Breast Cancer (Auckl) 2017;11:1178223417731565.
11. Markowitz GJ, Yang P, Fu J, et al. Inflammation-Dependent IL18 Signaling Restricts Hepatocellular Carcinoma Growth by Enhancing the Accumulation and Activity of Tumor-Infiltrating Lymphocytes. Cancer Res 2016;76(8):2394-2405.
Figures