0034
Liver Stiffness Measurement by Magnetic Resonance Elastography is not Affected by Hepatic Steatosis1Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Department of Radiology, Mayo Clinic, Rochester, MN, United States, 3Devision of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States, 4Devision of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN, United States
Synopsis
In a cross-sectional study, we investigated the relationship between pathology-assessed hepatic steatosis, MRI-assessed PDFF, and LSM with MRE in a large NAFLD population. No significant differences in LSM was found between patients with S1, S2, and S3 steatosis, and between all steatosis grades after patients were grouped according to fibrosis stage. After adjusting with fibrosis stage and age, there was no statistically significant relationship between liver stiffness and PDFF in patients with diagnosed steatosis (i.e., PDFF≥5%). LSM by MRE is not biased by increased liver fat content.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an emerging epidemic 1. Up to 25% of individuals with NAFLD progress to cirrhosis 2, and 7% of them to end-stage liver disease 3, demonstrating an increasing medical burden. As the histologic hallmark and pathogenic factor of NAFLD, hepatic steatosis is characterized by an excessive intra-cellular accumulation of fat within hepatocytes. Other accompanying pathological changes include inflammation, hepatocyte ballooning, and fibrosis 4. Among those pathologic features, fibrosis is the most important prognostic factor in predicting complications 5. Liver stiffness measurement (LSM) with magnetic resonance elastography (MRE) is currently the most accurate biomarker for noninvasive liver fibrosis evaluation 6. MRE assessment of liver fibrosis must take into account clinical information because other pathophysiologic conditions such as inflammation, venous congestion, portal hypertension, and cholestasis may affect liver stiffness 7. The presence of hepatic steatosis has been reported to affect liver stiffness as measured with ultrasound-based elastography 8-11. In contrast, most of MRE based studies have concluded that hepatic steatosis has no significant effect on MRE-assessed liver stiffness 12, 13. There are fundamental technical differences between ultrasound-based and MRE-based elastography that may account for these conclusions. In addition, most ultrasound-based studies have used histopathology-based assessment of steatosis, which is a subjective analysis and is associated with sampling variability and poor repeatability 14. The MRI-derived proton density fat fraction (PDFF) is a well-established imaging biomarker for quantifying liver steatosis on a continuous scale 15. The purpose of this study was to provide further evidence on the relationship between pathology-assessed hepatic steatosis, MRI-assessed PDFF, and MRE-assessed liver stiffness in a large patient cohort with either biopsy-proven NAFLD or at a high risk of NAFLD.Methods
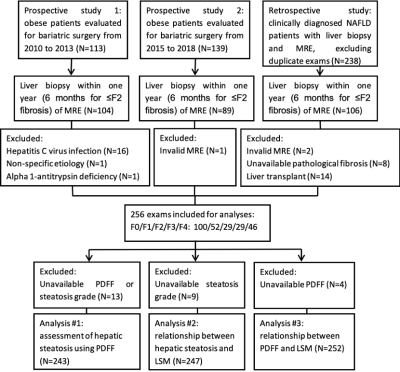
This retrospective study was approved by the Institutional Review Board, and written informed consent was waived. Patients with MRE and liver biopsy within one year (within 6 months for ≤F2 fibrosis) were initially identified. The final study cohort included 256 patients (Figure 1). LSM was retrieved from images using a 2D gradient-echo MRE sequence with imaging parameters set as previously described 16. PDFF was measured from 6-point Dixon imaging (IDEAL-IQ, GE Healthcare) if available, and from 2-point Dixon imaging otherwise, which were acquired using previously reported protocols 16, 17. Hepatic steatosis and fibrosis were assessed by histopathological grading/staging. First, we analyzed the diagnostic performance of PDFF for distinguishing hepatic steatosis with the receiver operating characteristic analyses. Second, variables influencing LSM were screened with univariant analyses, then identified with multivariable linear regression. Finally, the relationship between PDFF and LSM was adjusted with identified influencing factors, and the impact of PDFF on LSM was assessed with linear regression in all patients with diagnosed steatosis (PDFF≥5%), and in subgroups with 6-point Dixon PDFF, or with LSM<5 kPa.Results
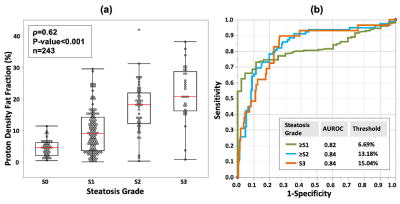
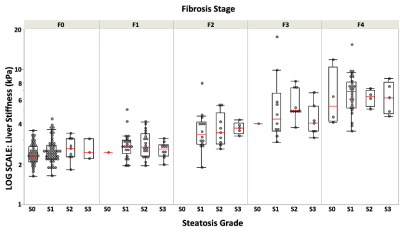
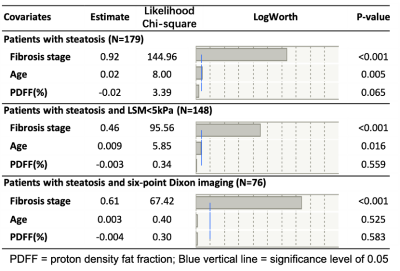
The diagnostic accuracies of PDFF in distinguishing different steatosis grade (S0-3) were all above 0.82 (Figure 2). No significant difference in LSM was found between patients with S1, S2, and S3 steatosis. After grouping subjects according to fibrosis stage, there was no significant difference in LSM between steatosis grades within each fibrosis stage (Figure 3). The multivariable linear regression indicated that only age (Estimate=0.02, p=0.018), hepatic fibrosis (Estimate=1.05, p<0.001), and steatosis (Estimate=-0.33, p=0.005) significantly influenced LSM. No statistically significant relationship was found between LSM and PDFF (Eestimate=-0.02, p=0.069) after adjusted with fibrosis stages and age in patients with diagnosed steatosis (Figure 4). Subgroup analyses in NAFLD patients with 6-point Dixon imaging, or with LSM<5kPa also revealed no significant relationship between LSM and PDFF (Estimate=-0.004 and -0.003, respectively, p>0.05 for both). Results of linear regressions were summarized in Table 1.Discussion
The results of this large cross-sectional study indicate that unlike ultrasound-based elastography techniques, liver stiffness measurements obtained with MRE are not significantly affected by the presence and severity of steatosis. After patients were stratified according to fibrosis stage, which is the primary cause of increased LSM, no significant difference was found in LSM between steatosis grades. In this study, PDFF showed high accuracy in distinguishing histopathology-based assessment of hepatic steatosis grade. Results of linear regressions suggested that the impact of PDFF on LSM was minimal and negligible compared to the contribution of fibrosis stage.Conclusion
The severity of hepatic steatosis has no significant influence on liver stiffness measurement with magnetic resonance elastography.Acknowledgements
This work has been supported by grant funding from the National Institutes of Health: R37 EB001981 (R.L.E.), R01 EB017197 (M.Y.), K23 DK115594 (A.M.A.), and the US Department of Defence: W81XWH-19-1-0583-01 (M.Y.).References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md.). 2016;64:73-84.
2. Önnerhag K, Nilsson PM, Lindgren S. Increased risk of cirrhosis and hepatocellular cancer during long-term follow-up of patients with biopsy-proven NAFLD. Scandinavian journal of gastroenterology. 2014;49:1111-1118.
3. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology (Baltimore, Md.). 2006;44:865-873.
4. Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V. Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clinics and research in hepatology and gastroenterology. 2011;35:23-28.
5. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e310.
6. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology (Baltimore, Md.). 2017;66:1486-1501.
7. Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 2, Diagnostic Performance, Confounders, and Future Directions. AJR Am J Roentgenol. 2015;205:33-40.
8. Petta S, Maida M, Macaluso FS, et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.). 2015;62:1101-1110.
9. Shen F, Mi YQ, Xu L, et al. Moderate to severe hepatic steatosis leads to overestimation of liver stiffness measurement in chronic hepatitis B patients without significant fibrosis. Alimentary pharmacology & therapeutics. 2019;50:93-102.
10. Karlas T, Petroff D, Sasso M, et al. Impact of controlled attenuation parameter on detecting fibrosis using liver stiffness measurement. Alimentary pharmacology & therapeutics. 2018;47:989-1000.
11. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730.
12. Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756.
13. Leitão HS, Doblas S, Garteiser P, et al. Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology. 2017;283:98-107.
14. Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology.2005;128:1898-1906.
15. Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magnetic resonance imaging clinics of North America.2010;18:337-357, ix.
16. Chen J, Yin M, Talwalkar JA, et al. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology. 2017;283:418-428.
17. Allen AM, Shah VH, Therneau TM, et al. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery. Hepatology (Baltimore, Md.). 2020;71:510-521
Figures