0026
Low Rank Motion Correction for free breathing first pass myocardial perfusion1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Conventional myocardial perfusion imaging requires free breathing acquisitions, with high spatial and temporal resolution. Leveraging the underlying redundant anatomic information to improve this multi-contrast application is challenging due to motion. Here a novel Low Rank Motion Corrected (LRMC) reconstruction is proposed to enable highly accelerated, motion corrected free breathing first pass myocardial perfusion imaging. This approach combines low rank subspace modelling (to resolve contrast) and non-rigid motion fields (to correct respiratory motion). The proposed approach successfully corrects respiratory motion and considerably improves image quality compared to conventional iterative SENSE reconstruction.
INTRODUCTION:
Myocardial perfusion imaging is conventionally acquired with multiple slices in multiple cardiac phases, covering the temporal evolution of a contrast agent after administration. Due to the long acquisition times (up to 60s), free breathing is often required. Respiratory motion can affect the interpretation of the images, introduce artefacts and interfere with automated algorithms for quantitative perfusion. Reconstruction methods such as k-t SLR1 have been proposed to improve image quality in myocardial perfusion imaging, however they are sensitive to motion. Other methods such as MASTeR2 incorporate motion into the regularization, but do not fully leverage redundant information along multiple contrasts. Recently Multitasking3 has provided a framework to simultaneously resolve motion and contrast as separable dimensions of a high order tensor problem, enabling low rank subspace basis in the presence of motion in an efficient framework, however this approach does not exploit local redundancies at a patch level. Here we combine low rank models4,5,6 with generalized motion correction7,8,9 in a Low Rank Motion Corrected (LRMC) reconstruction, regularized with a high-dimensionality patch-based reconstruction (HD-PROST)10 to correct for respiratory motion and considerably improve myocardial perfusion image quality.METHODS:
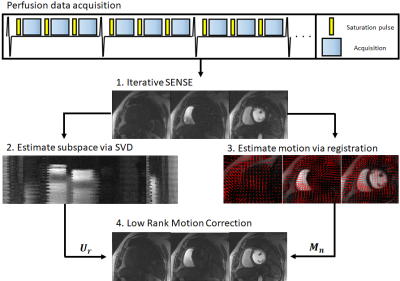
The proposed framework can be divided in four steps: 1) auxiliary iterative SENSE reconstruction; 2) low rank subspace estimation; 3) respiratory motion estimation; 4) Low Rank Motion Correction (Fig.1). A preliminary iterative SENSE (itSENSE)11 reconstruction is performed. A low rank subspace is estimated via a singular value decomposition of these reconstructed images, assembled in a Casorati matrix. From the same auxiliary images, respiratory motion fields are obtained via free-form deformations image registration.12,13 Finally, the low rank operator and motion fields are combined in the proposed LRMC reconstruction:$$ L(y , T_b)= argmin_{y , T_b} ||Σ_nA_nFCM_nU_ry-k||_2^2 + \lambda Σ_b ||T_b||_* , s.t. T_b=Q_b(y)$$
where $$$A_n$$$ is the corresponding sampling trajectory for the n-th temporal frame, $$$F$$$ is the Fourier transform, $$$C$$$ are coil sensitivities, $$$M_n$$$ are the motion fields, $$$y$$$ are singular value images, $$$U_r$$$ is the low rank compression (obtained by truncation of the left singular vectors to rank r), $$$k$$$ is the acquired k-space data and $$$Q_b$$$ assembles a 3D HD-PROST tensor around a pixel b, similar patches to the patch around b and along the multiple singular contrast dimensions.
EXPERIMENTS:
Five patients referred for a clinical cardiac MR scan were scanned on a 3T scanner (Philips Achieva). Imaging parameters included field of view (FOV) = 256x256 mm2; 10 mm slice thickness; resolution = 2x2 mm2; TE/TR = 1.6/3.5 ms; radial golden angle; 3 slices (in 3 cardiac phases); flip angle 15º; WET saturation pulse; 100 ms saturation delay; nominal scan time ~60s. Data was reconstructed with itSENSE and with LRMC. For the proposed LRMC, each separate frame constituted a separate motion state (i.e. no binning) and r = 10 was considered.RESULTS:
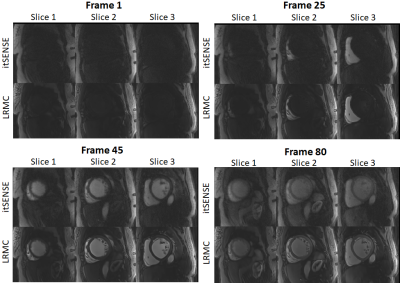
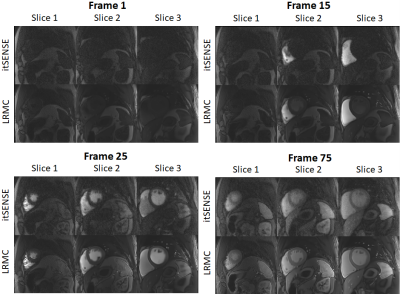
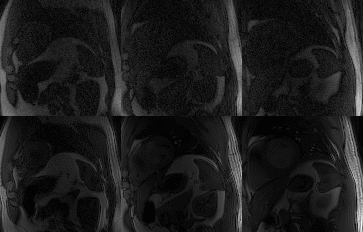
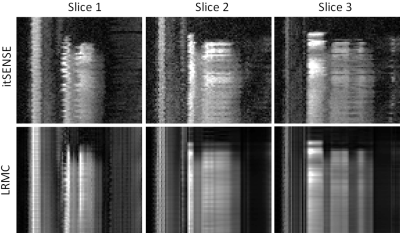
Myocardial perfusion frames for two representative subjects are shown in Fig.2 and Fig.3. Substantial streaking artefacts, blurring and noise amplification is present with conventional itSENSE in addition to respiratory motion. The proposed LRMC corrects for the respiratory motion and achieves higher apparent SNR and sharpness with less residual artefacts. An animation of the first pass perfusion in another representative subject is shown in Fig.4. Here, it can be seen that a motion corrected series with substantially improved image quality is achieved with LRMC, while capturing all the dynamics of the first pass. Moreover, cardiac motion artifacts can be observed in the itSENSE reconstruction (possibly due to arrythmias or fast/varying heartrate). The proposed approach considers each frame as a separate motion state (i.e. no binning is performed), therefore correcting for any remaining cardiac motion along with respiratory motion. A 1D temporal profile for the same representative subject is shown in Fig.5. Again, both respiratory and cardiac motion (in addition to residual artefacts/noise) are observed in the itSENSE reconstruction; all of these are corrected for with the proposed LRMC.CONCLUSION:
A novel Low Rank Motion Corrected reconstruction is employed to enable free breathing first pass myocardial perfusion imaging with superior image quality. A considerable increase in image quality (compared to itSENSE) is observed, along with respiratory (and remaining cardiac) motion correction. The proposed approach could enable higher spatio-temporal perfusion imaging, non-ECG triggered perfusion and better automated quantitative perfusion. This will be investigated in future work.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC (EP/P001009, EP/P032311/1, EP/P007619/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Lingala SG, Hu Y, DiBella E, Jacob M. Accelerated dynamic MRI exploiting sparsity and low-rank structure: kt SLR. IEEE transactions on medical imaging. 2011 Jan 31;30(5):1042-54.
2. Asif MS, Hamilton L, Brummer M, Romberg J. Motion‐adaptive spatio‐temporal regularization for accelerated dynamic MRI. Magnetic Resonance in Medicine. 2013 Sep;70(3):800-12.
3. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature biomedical engineering. 2018 Apr;2(4):215-26.
4. McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans. Med. Imaging 2014;33:2311–2322 doi: 10.1109/TMI.2014.2337321.
5. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
6. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
7. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 2005;54:1273–1280 doi: 10.1002/mrm.20656.
8. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
9. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion‐corrected 3D whole‐heart coronary vessel wall imaging. Magnetic resonance in medicine. 2017 May;77(5):1894-908.
10. Bustin A, Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High - dimensionality undersampled patch - based reconstruction ( HD - PROST ) for accelerated multi - contrast MRI. Magn. Reson. Med. 2019:3705–3719 doi: 10.1002/mrm.27694.
11. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Oct;46(4):638-51.
12. Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE transactions on medical imaging. 1999 Aug;18(8):712-21.
13. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, Ourselin S. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine. 2010 Jun 1;98(3):278-84.
Figures