0024
Left Ventricular Myocardial Stiffness Decreases after Stem Cell Therapy in Amyloidosis Patients: Monitored with Cardiac MR Elastography
Arvin Arani1, Jessica Magnuson1, Joshua D. Trzasko1, Yi Sui1, Kevin J. Glaser1, Armando Manduca1, Richard L. Ehman1, Sudhakar K. Venkatesh1, and Philip A. Araoz1
1Mayo Clinic, Rochester, MN, United States
1Mayo Clinic, Rochester, MN, United States
Synopsis
Light chain (AL) cardiac amyloidosis is a disease where abnormal proteins are deposited in the heart tissue, commonly resulting in elevated myocardial stiffness. In cases with poor prognosis, autologous hematopoietic stem cell transplantation is used as a therapy. However, organ response monitoring is currently limited and challenging. The goal of this study is to evaluate the feasibility of using cardiac magnetic resonance elastography (MRE) to monitor changes in left ventricular (LV) myocardial stiffness after stem cell transplantation for treatment of AL amyloidosis. MRE detected a statistically significant (p=0.007) decrease in LV stiffness (mean decrease: 1.6±0.7 kPa, 17.0±7.4%) post therapy.
Introduction
Amyloidosis is a disease in which abnormal proteins infiltrate organs, causing dysfunction. Amyloid is categorized by the type of protein and its folding. One of the most common types of amyloidosis is amyloid light chain (AL) amyloidosis. In AL amyloidosis plasma cell dyscrasias create excess immunoglobulin light chain fragments, which form abnormal proteins which deposit in tissues (1). The worst prognosis in AL amyloidosis occurs with infiltration of the heart, which leads to myocardial thickening and heart failure (2). The definitive treatment of AL amyloidosis is autologous hematopoietic stem cell transplantation, which prevents further deposition of abnormal proteins. Organ response and progression is currently monitored with multiple, limited, insensitive, non-specific biomarkers. Amyloid infiltration is widely accepted to cause increased tissue stiffness (3), including within the heart. Dysregulation of myocardial stiffness plays an important role in cardiac function and can lead to congestive heart failure (4), contribute to left ventricular (LV) remodeling, and a significant challenge in treating myocardial infarction (5). Shear wave elastography is an emerging imaging approach for measuring myocardial stiffness in vivo (6-15). Recently, cardiac magnetic resonance elastography (MRE) reported that patients with cardiac amyloidosis have significantly elevated myocardial stiffness (14) compared to healthy age-matched controls. The goal of this study is to evaluate the feasibility of using cardiac magnetic resonance elastography (MRE) to monitor changes in left ventricular myocardial stiffness after stem cell transplantation for treatment of AL amyloidosis.Methods
Five patients with AL cardiac amyloidosis were enrolled in this study prior to undergoing hematopoietic stem cell transplantation, and after receiving institutional review board and written informed consent approval. All subjects underwent cardiac MRI/MRE prior to therapy (baseline) and at their scheduled approximate 3 month follow-up visit. The mean age at time of enrollment was 58 (median: 59, max: 66, min: 51), with a mean follow-up time of 117.6 days (median: 114, max: 130, min: 106). MRE imaging was conducted at a vibration frequency of 140 Hz using the same procedure as previously described (16). Exams with an octahedral shear strain signal-to-noise ratio (OSS-SNR) (17,18) above 1.1 were considered in the analysis. A paired Student’s t-test was performed on the mean left ventricular myocardial stiffness at baseline and follow-up. A p-value of less than 0.05 was considered statistically significant. The estimated percentage change in myocardial stiffness per 100 days was calculated to help compensate for the slight differences in follow-up times.Results
Systolic center-slice magnitude and elastogram images for all 5 volunteers at baseline and at their follow-up visit are shown in Figure 1. Quantitative stiffness maps demonstrate the decrease in left-ventricular myocardial stiffness post-therapy in all 5 patients. Quantitative mean LV myocardial stiffness values at baseline and at follow-up are plotted in Figure 2. Myocardial stiffness as a function of days after each patent’s first imaging session is plotted in Figure 3. The measured percentage change in stiffness at follow-up and the calculated expected change per 100 days post therapy have been plotted in Figure 4. The mean LV myocardial stiffness decreased by 17.0±7.4% (median: 15.1%, max: 28.4%, min: 8.2%) at follow-up (14.4±5.9% per 100 days), across patients. The mean absolute change in myocardial stiffness was calculated to be -1.6±0.7 kPa (median: -1.3 kPa, max: -2.4 kPa, min: -0.9 kPa) from baseline to follow-up. This decrease in stiffness was found to statistically significant (p = 0.007).Discussion and Conclusions
This study demonstrates there is a statistically significant (p=0.007) decrease in LV myocardial stiffness (mean: 1.6±0.7 kPa, 17.0±7.4%) post hematopoietic stem cell transplantation as measured by MRE. These results not only suggest that stem cell therapy is affecting the biomechanics of the heart, but also that CMRE is a sensitive non-invasive monitoring technique capable of detecting these changes within a short follow-up time (~117 days post-therapy). These results motivate future investigations of cardiac MRE in larger patient cohorts, to monitor treatment response, and potentially as a means to detect early stages of cardiac diseases.Acknowledgements
We would like to thank Kathy Brown for recruiting and scheduling all patient exams. This work was supported by the National Institutes of Health grants K12HD65987-11 and by internal grants funded by Mayo Clinic, Department of Radiology.References
1. Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Reviews 2012;26(4):137-154.2. Sher T, Gertz MA. Recent advances in the diagnosis and management of cardiac amyloidosis. Future Cardiology 2014;10(1):131-146.
3. Fitzpatrick AW, Park ST, Zewail AH. Exceptional rigidity and biomechanics of amyloid revealed by 4D electron microscopy. Proceedings of the National Academy of Sciences of the United States of America 2013;110(27):10976-10981.
4. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. The New England journal of medicine 2004;350(19):1953-1959. 5. Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annual review of biomedical engineering 2005;7:223-253.
6. Kolipaka A, Aggarwal SR, McGee KP, Anavekar N, Manduca A, Ehman RL, Araoz PA. Magnetic resonance elastography as a method to estimate myocardial contractility. Journal of magnetic resonance imaging : JMRI 2012;36(1):120-127.
7. Kolipaka A, Mcgee KP, Araoz PA, Glaser KJ, Manduca A, Romano AJ, Ehman RL. MR Elastography as a Method for the Assessment of Myocardial Stiffness: Comparison with an Established Pressure-Volume Model in a Left Ventricular Model of the Heart. Magnet Reson Med 2009;62(1):135-140. 8. Elgeti T, Beling M, Hamm B, Braun J, Sack I. Cardiac magnetic resonance elastography: toward the diagnosis of abnormal myocardial relaxation. Investigative radiology 2010;45(12):782-787.
9. Elgeti T, Knebel F, Hattasch R, Hamm B, Braun J, Sack I. Shear-wave amplitudes measured with cardiac MR elastography for diagnosis of diastolic dysfunction. Radiology 2014;271(3):681-687. 10. Elgeti T, Laule M, Kaufels N, Schnorr J, Hamm B, Samani A, Braun J, Sack I. Cardiac MR elastography: comparison with left ventricular pressure measurement. Journal of cardiovascular magnetic resonance 2009;11:44.
11. Couade M, Pernot M, Messas E, Bel A, Ba M, Hagege A, Fink M, Tanter M. In vivo quantitative mapping of myocardial stiffening and transmural anisotropy during the cardiac cycle. IEEE transactions on medical imaging 2011;30(2):295-305.
12. Song P, Zhao H, Urban M, Manduca A, Pislaru S, Kinnick R, Pislaru C, Greenleaf J, Chen S. Improved Shear Wave Motion Detection Using Pulse-Inversion Harmonic Imaging with a Phased Array Transducer. IEEE transactions on medical imaging 2013;32(12):2299-2310.
13. Hollender PJ, Wolf PD, Goswami R, Trahey GE. Intracardiac echocardiography measurement of dynamic myocardial stiffness with shear wave velocimetry. Ultrasound in medicine & biology 2012;38(7):1271-1283.
14. Arani A, Arunachalam SP, Chang ICY, Baffour F, Rossman PJ, Glaser KJ, Trzasko JD, McGee KP, Manduca A, Grogan M, Dispenzieri A, Ehman RL, Araoz PA. Cardiac MR elastography for quantitative assessment of elevated myocardial stiffness in cardiac amyloidosis. Journal of magnetic resonance imaging : JMRI 2017;46(5):1361-1367.
15. Wassenaar PA, Eleswarpu CN, Schroeder SA, Mo X, Raterman BD, White RD, Kolipaka A. Measuring age-dependent myocardial stiffness across the cardiac cycle using MR elastography: A reproducibility study. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2016;75(4):1586-1593.
16. Arani A, Glaser KL, Arunachalam SP, Rossman PJ, Lake DS, Trzasko JD, Manduca A, McGee KP, Ehman RL, Araoz PA. In vivo, high-frequency three-dimensional cardiac MR elastography: Feasibility in normal volunteers. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2016.
17. McGarry MD, Van Houten EE, Perrinez PR, Pattison AJ, Weaver JB, Paulsen KD. An octahedral shear strain-based measure of SNR for 3D MR elastography. Physics in medicine and biology 2011;56(13):N153-164.
18. Arani A, Glaser KL, Arunachalam SP, Rossman PJ, Lake DS, Trzasko JD, Manduca A, McGee KP, Ehman RL, Araoz PA. In vivo, high-frequency three-dimensional cardiac MR elastography: Feasibility in normal volunteers. Magnet Reson Med 2017;77(1):351-360.
Figures
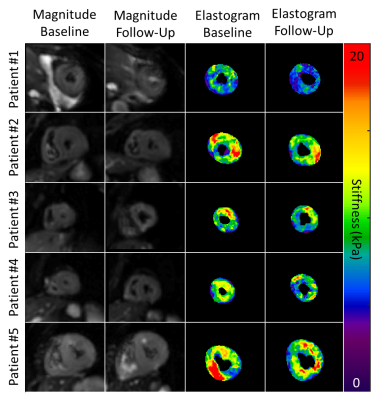
Systolic center-slice
magnitude and elastogram images for all 5 volunteers at baseline and at their
follow-up visit. Quantitative stiffness maps demonstrate the decrease in
left-ventricular myocardial stiffness post-therapy.
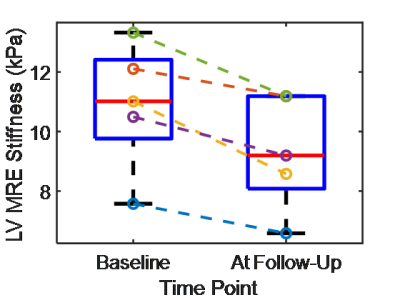
Quantitative mean left
ventricular myocardial stiffness values at baseline and at follow-up after post
stem cell therapy for all 5 patients.
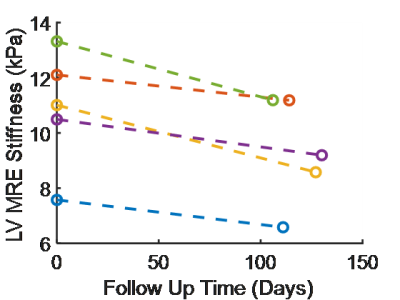
Myocardial stiffness at
baseline and as a function of days after initial imaging session.
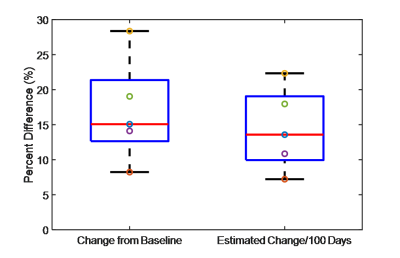
The measured percentage
change in stiffness at follow-up and the calculated expected change per 100
days post baseline. The estimated change
per 100 days was calculated to help compensate for the slight differences in
follow-up times.