0023
Increased Cardiac Pi in the Diabetic Heart Observed Using STEAM 31P MRS at 7T
Ladislav Valkovic1,2, Andrew Apps1, Jane Ellis1, Damian J Tyler1,3, Stefan Neubauer1, Albrecht Ingo Schmid4, Oliver J Rider1, and Christopher T Rodgers1,5
1Oxford Centre for Clinical Magnetic Resonance Research (OCMR), RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 4High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 5Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
1Oxford Centre for Clinical Magnetic Resonance Research (OCMR), RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 4High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 5Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
Synopsis
Impaired cardiac energetics are characterized by a reduced phosphocreatine to adenosine-triphosphate ratio (PCr/ATP), however, changes in inorganic phosphate (Pi) may impact the Gibbs energy of ATP hydrolysis earlier in the disease process. Quantifying this in the diabetic heart may help explain latent diastolic dysfunction. Therefore, we used STEAM 31P-MRS at 7T to measure Pi/PCr in a type 2 diabetic (T2DM) cohort, and demonstrated an increased Pi/PCr in the diabetic human heart in comparison to healthy subjects. No correlation between PCr/ATP and Pi/PCr hints that multiple mechanisms contribute to these perturbations with candidates including impairment of CK flux and substrate inflexibility.
Introduction
Impaired cardiac energetics are characterized by a reduced phosphocreatine to adenosine-triphosphate ratio (PCr/ATP) which is exacerbated by exertion is a hallmark of the diabetic heart1. This may contribute to diastolic dysfunction, which is commonly regarded as a precursor to heart failure in this patient group2. However, the mechanism remains unclear. Energy homeostasis maintains ATP until late in heart failure3, however, changes in inorganic phosphate (Pi) may impact the Gibbs energy of ATP hydrolysis earlier in the disease process. Quantifying this in the diabetic heart may therefore help explain latent diastolic dysfunction. STEAM phosphorus MR spectroscopy (31P-MRS) at 7T with long mixing time (TM) was recently shown to reliably resolve Pi without blood pool 2,3-DPG contamination4.Therefore, in this study we sought to use STEAM 31P-MRS to measure Pi/PCr in a type 2 diabetic (T2DM) cohort and compare these with healthy controls.
Methods
17 T2DM patients (3F, 61 ± 7 years, BMI 27.1 ± 4.2 kg/m2, HbA1c 7.2 ± 1.2%, left ventricular ejection fraction (LVEF) 59 ± 4%, all on oral medications only), and 23 controls (9F, 43 ± 16 years, BMI 24.1 ± 2.6 kg/m2) were recruited for this study. MRS was performed using a 1H loop for localizers and a 16-channel receive array with surface transmit loop (both RAPID Biomedical, Rimpar, Germany) for 31P-MRS at 7T (Magnetom, Siemens Healthineers, Erlangen Germany). PCr/ATP was acquired at rest using UTE-CSI (TR 2.2 s, nominal voxel size 30 × 15 × 33 mm3). Pi/PCr was acquired from the septum using interleaved STEAM 31P-MRS acquisitions MRS4 (TR 6 s, TE 13 ms, TM PCr: 7/Pi 60 ms, Figure 1). We have also assessed diastolic function in diabetics by assessment of mitral annular tissue Doppler velocities, in particular as a ratio between early mitral inflow velocity and mitral annular early diastolic velocity (E/e').MRS data were analzsed using the time domain fitting AMARES routine implemented in the OXSA toolbox5. Data were corrected for partial saturation using literature relaxation times6, 7. PCr/ATP was calculated using the γ-ATP peak, since β-ATP is outside the excitation bandwidth of the used pulse. Intramyocardial pH was calculated from the STEAM data using the Henderson-Hasselbalch equation8. Student t-test was used to determine statistically significant (p<0.05) differences between the subject groups.
Results and Discussion
Typical STEAM 31P-MRS spectra from a control and a diabetic heart are depicted in Figure 2. Measured cardiac Pi/PCr was significantly higher in T2DM patients (0.13 ± 0.05 vs. 0.10 ± 0.03 p = 0.03), mirroring a decrease in PCr/ATP (1.70 ± 0.31 vs 2.07 ± 0.39 p < 0.01, Figure 3). The increased Pi in the hearts of T2D patients will limit the available energy from ATP hydrolysis. If this progresses, diastolic and ultimately systolic function will become impaired.Our reported values of Pi/PCr in healthy volunteers are in good agreement with literature4, 9, and while the T2DM Pi/PCr values are increased, they are still lower than the previously reported Pi/PCr in patients with cardiomyopathies7, 9. This further suggests slowly deteriorating cardiac energetics in T2DM. We did not observe any significant differences in the myocardial pH between the groups 7.14 ± 0.12 (T2DM) vs 7.10 ± 0.12 (controls) p = 0.31. This is in agreement with reports in patients with cardiomyopathies9, 10.
Diastolic dysfunction, indicated by raised E/e’ (mean) didn’t correlate with Pi/PCr (R2 = 0.08, p = 0.43), but did negatively correlate with PCr/ATP (R2 = 0.33, p = 0.02) and weakly with Pi/ATP (R2 = 0.26, p = 0.12), as shown in Figure 4. We did not observe any correlation (p = 0.57) between cardiac PCr/ATP and Pi/PCr (Figure 5), potentially suggesting the rise in Pi may be mechanistically independent to the fall in PCr/ATP, with candidates including impairment of CK flux and substrate inflexibility11.
Conclusion
Using STEAM 31P-MRS at 7T we have demonstrated for the first time increased Pi/PCr in the diabetic human heart in comparison to healthy subjects. No correlation between measured PCr/ATP and Pi/PCr hints that multiple mechanisms contribute to these perturbations with candidates including impairment of CK flux and substrate inflexibility.Acknowledgements
CTR and LV are funded by a Sir Henry Dale Fellowship from the Wellcome Trust [098436/Z/12/B]. AA is funded by a British Heart Foundation clinical research training fellowship [FS/17/18/32449]. AIS is funded by the Austrian Science Fund (FWF) Schrödinger Fellowship J 4043. Support of the Slovak Grant Agencies VEGA [2/0003/20] and APVV [#19–0032] is also gratefully acknowledged.References
- Levelt, E., C.T. Rodgers, et al., Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J, 2016. 37(46): p. 3461-3469.
- From, A.M., C.G. Scott, and H.H. Chen, The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol, 2010. 55(4): p. 300-5.
- Beer, M., T. Seyfarth, et al., Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol, 2002. 40(7): p. 1267-74.
- Apps, A., L. Valkovič, et al., Quantifying the effect of dobutamine stress on myocardial Pi and pH in healthy volunteers: A (31) P MRS study at 7T. Magn Reson Med, 2021. 85(3): p. 1147-1159.
- Purvis, L.A.B., W.T. Clarke, et al., OXSA: An open-source magnetic resonance spectroscopy analysis toolbox in MATLAB. PLoS One, 2017. 12(9): p. e0185356.
- Rodgers, C.T., W.T. Clarke, et al., Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla. Magn Reson Med, 2014. 72(2): p. 304-15.
- Valkovič, L., W.T. Clarke, et al., Measuring inorganic phosphate and intracellular pH in the healthy and hypertrophic cardiomyopathy hearts by in vivo 7T (31)P-cardiovascular magnetic resonance spectroscopy. J Cardiovasc Magn Reson, 2019. 21(1): p. 19.
- Bailey, I.A., S.R. Williams, et al., Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem J, 1981. 196(1): p. 171-8.
- Jung, W.I., L. Sieverding, et al., 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation, 1998. 97(25): p. 2536-42.
- de Roos, A., J. Doornbos, et al., Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: assessment with proton-decoupled P-31 MR spectroscopy. J Magn Reson Imaging, 1992. 2(6): p. 711-9.
- Rider, O.J., A. Apps, et al., Non-Invasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized (13)C MRI. Circ Res, 2020.
Figures
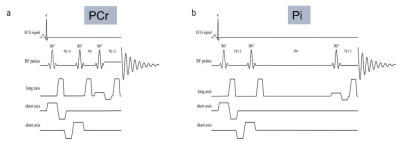
Figure 1 STEAM 31P-MRS sequence for interleaved RF excitation centred at PCr (a: RF excitation centred at 0ppm relative to PCr, TM 7ms) and Pi (b: RF excitation centred at 5 ppm relative to PCr, TM 60 ms).
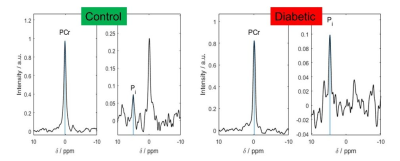
Figure 2 Typical
STEAM PCr and Pi spectra from a control (left) and a T2DM (right) participant.
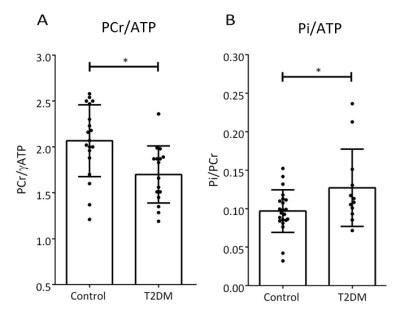
Figure 3 Energetics
in controls and diabetics. PCr/ATP was significantly lower (p < 0.01) and
Pi/ATP was significantly higher (p = 0.03) in T2DM patients. Please note that due
to myocardial [Pi] being low, and BMI of some participants being well over
30 kg/m2 Pi was reliably quantifiable4, i.e.
SNR>3, in 13/17 T2DM, and 20/23 control STEAM spectra.
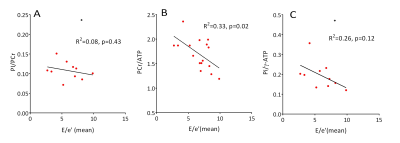
Figure 4 The
correlation of diastolic function with different measures of myocardial
energetics in diabetics. The black
dots in A and C is defined as outliers by Grubbs test (p < 0.05)
and are excluded from the correlation calculation.
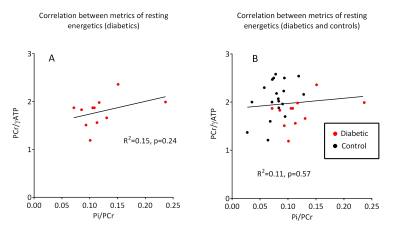
Figure 5 Correlation between cardiac metabolism metrics of rest energetics
in diabetics