0022
Effect of doxorubicin treatment on myocardial metabolism in patients with breast cancer1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Electrical and Computer Engineering, University of Texas at Dallas, Richardson, TX, United States, 4GE Healthcare, Dallas, TX, United States, 5Pharmacy Practice, Texas Tech University, Dallas, TX, United States, 6Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 7Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
This study evaluates the feasibility of hyperpolarized 13C MRS for detection of early metabolic response in the myocardium of patients after anthracycline treatment. Patients with breast cancer were studied using hyperpolarized [1-13C]pyruvate before and after doxorubicin. Appearance of [13C]bicarbonate was significantly decreased after doxorubicin compared to the baseline exam while no change in [1-13C]lactate was detected. The reproducibility of hyperpolarized exams was examined by two injections of hyperpolarized [1-13C]pyruvate. The study demonstrates that hyperpolarized 13C spectra of the heart are sensitive to early injury of the mitochondria after doxorubicin therapy in patients with breast cancer in a reproducible manner.
Background
Chemotherapy with anthracycline is restricted by an increased risk of cardiotoxicity, resulting in downstream mitochondrial damage and impaired energy production. More than 5% of women requiring treatment for breast cancer with anthracyclines develop heart disease (1). Several lines of research point to mitochondrial damage as a root mechanism of toxic cardiomyopathy. The mechanism of cardiac injury has been studied extensively (2-9), evidence converging towards mitochondrial damage with decreased energetic metabolism and increased oxidative stress, but its molecular basis is not fully understood. Early detection of myocardial injury due to anthracyclines with implementation of targeted therapies is an important but unrealized objective (10). HP 13C MRS and imaging have been introduced as a method to detect fluxes through individual enzyme-catalyzed reactions such as pyruvate dehydrogenase (PDH) and lactate dehydrogenase (LDH) (11-14). Since PDH is integral to normal function of mitochondria, this technology is a promising approach for serial exams in patients with the potential of tailoring maximal doxorubicin to the patient based on early signs of cardiotoxicity. However, repeated HP studies have not been performed in women with breast cancer, and little is known about the reproducibility of HP exams. This study was designed to test the hypothesis that exams of cardiac metabolism using HP [1-13C]pyruvate are feasible before and after conventional chemotherapy in women with breast cancer, and that production of HP [13C]bicarbonate or HP [1-13C]lactate may be sensitive to chemotherapy (15).Methods
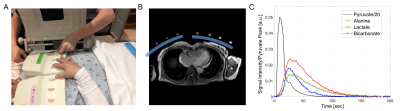
Of 562 consecutive patients with biopsy proven human epidermal growth factor receptor 2 (HER2) receptor negative breast cancer screened for eligibility nine patients completed HP studies before and after therapeutic protocol with doxorubicin (4 cycles of doxorubicin and cyclophosphamide). All MR studies were performed on a clinical 3T MR scanner (GE Healthcare Discovery 750w). A clamshell transmit coil and a pair of 4-channel receive arrays were used for 13C acquisition. The receive coils configuration relative to the heart in axial plane, and a representative kinetic data set are shown in fig. 1. 13C data were acquired from a 10-cm long-axis slice using a cardiac-gated pulse and acquire sequence ~600 ms after the previous R wave, corresponding to cardiac diastasis. Data were acquired from all 8 channels 6 seconds from the beginning of the pyruvate injection using a slice-selective RF pulse estimated to be a 10° excitation, every 2.8 to 3.8 seconds for approximately 4 minutes (80 timepoints), depending on the cardiac rate. To examine the reproducibility of HP data acquisition during the same session five patients before chemotherapy and two patients after chemotherapy had two injections of HP [1-13C]pyruvate separated by 30 min. After clearing the intravenous line with saline, one mL of blood was drawn at baseline and every 5 to 15 minutes after the bolus of hyperpolarized pyruvate. Concentrations of 13C and 12C pyruvate, glucose and lactate were determined using LC-MS/MS.Results and Discussion
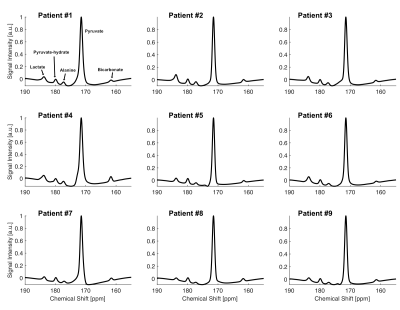
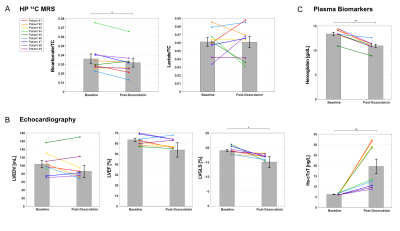
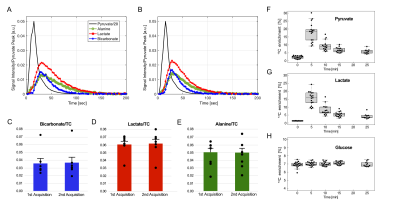
All participants tolerated the HP exam well and no adverse effects were detected over one-hour monitoring period. Baseline, summed 13C NMR spectra for all nine patients (fig. 2), were similar, all showing HP [13C]bicarbonate, [1-13C]lactate, [1-13C]alanine, [1-13C]pyruvate-hydrate and [1-13C]pyruvate. The fraction of [13C]bicarbonate, [1-13C]lactate, and [1-13C]alanine, relative to total 13C signal (TC) was 0.036 ± 0.005, 0.448 ± 0.023, and 0.045 ± 0.004, respectively. The production of HP [13C]bicarbonate and HP [1-13C]lactate at baseline and after doxorubicin therapy is shown for each patient in fig. 3. There was a significant decrease in HP [13C]bicarbonate relative to TC signal (bicarbonate/TC = 0.032 ± 0.005, p = 0.037) after doxorubicin therapy compared to baseline. However, there was no change in HP [1-13C]lactate (lactate/TC = 0.44 ± 0.04, P = 0.9) or [1-13C]alanine (alanine/TC = 0.045 ± 0.006, P = 0.9). After therapy, patients had significant changes in hemoglobin, high-sensitivity troponin (hs-cTnT) and peak left ventricular global longitudinal strain (LVGLS).As illustrated in fig. 4, [13C]bicarbonate/TC (0.037 ± 0.007 for 1st injection and 0.038 ± 0.007 for 2nd injection, p = 0.743), [1-13C]lactate/TC (0.061 ± 0.005 vs. 0.062 ± 0.006, p = 0.5), and [1-13C]alanine/TC (0.051 ± 0.005 vs. 0.050 ± 0.005, P = 0.8) were not different between the first and second study. The effects of the bolus of HP [1-13C]pyruvate on the fractional enrichment of circulating pyruvate in plasma is shown in fig. 4F-H. Enrichment of [1-13C]pyruvate increased to 18.9 ± 0.02 % at 5-min post a bolus injection of [1-13C]pyruvate and subsequently decreased towards natural 13C abundance level. 13C enrichment of [1-13C]lactate was also observed 5-min post injection with a similar subsequent decrease. No enrichment of [13C]glucose, [2-13C]lactate, or [3-13C]lactate was observed and concentrations remained in line with natural 13C abundance.
Conclusion
In summary, the current study demonstrates that 1) myocardial HP 13C spectra are readily acquired and are sensitive to cardiotoxic chemotherapy in patients treated for breast cancer, 2) data are reproducible within the same visit and 3) serial exams are feasible and well-tolerated by all participants. Although this novel approach will need to be further replicated at a larger scale for generalizability, these results indicate that detection of early mitochondrial impairment in cardiomyopathy is feasible, safe and reproducible, an important step towards early diagnosis and personalized therapy before irreversible heart disease.Acknowledgements
Personnel Support: We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern for imaging the volunteers – Jeannie Baxter, RN, Kelley Derner, RN, Salvador Pena, Corey Mozingo, Maida Tai, and Richard Martin.Funding: The Cancer Prevention and Research Institute of Texas (RP180404); The Texas Institute for Brain Injury and Repair; National Institutes of Health of the United States (P41 EB015908, S10 RR029119, S10 OD018468, R01 NS107409); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award (UTD 1907789)References
1. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018 Feb 20;137(8):e30–e66. PMCID: PMC6722327
2. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. Springer US; 2017 Feb;31(1):63–75. PMCID: PMC5346598
3. Stěrba M, Popelová O, Vávrová A, Jirkovský E, Kovaříková P, Geršl V, et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal. 2013 Mar 10;18(8):899–929. PMCID: PMC3557437
4. Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. Nature Publishing Group; 2012 Nov;18(11):1639–42.
5. Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015 Nov;309(9):H1453–67. PMCID: PMC4666974
6. Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A, et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation. 2016 Apr 26;133(17):1668–87. PMCID: PMC4856587
7. Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation. Lippincott Williams & Wilkins; 2007 Jul 31;116(5):506–14.
8. Montaigne D, Marechal X, Preau S, Baccouch R, Modine T, Fayad G, et al. Doxorubicin induces mitochondrial permeability transition and contractile dysfunction in the human myocardium. Mitochondrion. 2011 Jan;11(1):22–6.
9. Ky B, Vejpongsa P, Yeh ETH, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ. Res. Lippincott Williams & Wilkins Hagerstown, MD; 2013 Aug 30;113(6):754–64. PMCID: PMC4046703
10. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. Lippincott Williams & Wilkins Hagerstown, MD; 2015 Jun 2;131(22):1981–8.
11. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med. 2013 Aug 14;5(198):198ra108. PMCID: PMC4201045
12. Rider OJ, Apps A, Miller JJ, Lau JY, Lewis AJ, Peterzan MA, et al. Non-Invasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized 13C MRI. Circ. Res. American Heart Association Bethesda, MD; 2020 Feb 4;100(6):10158–736. PMCID: PMC7077975
13. Gallagher FA, Woitek R, McLean MA, Gill AB, Manzano Garcia R, Provenzano E, et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci USA. 2020 Jan 28;117(4):2092–8. PMCID: PMC6995024
14. Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ. Res. 2016 Sep 15;119(11):1177–82. PMCID: PMC5102279
15. Park JM, Reed GD, Liticker J, Putnam WC, Chandra A, Yaros K, et al. Effect of Doxorubicin on Myocardial Bicarbonate Production from Pyruvate Dehydrogenase in Women with Breast Cancer. Circ. Res. 2020 Dec;104:19773.
Figures