0012
A nomogram combining T2WI-based radiomics features and clinical variables for prediction of neoadjuvant chemotherapy response in osteosarcoma1Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China, 2Department of Imaging, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 3Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China, 4Department of Pathology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
Synopsis
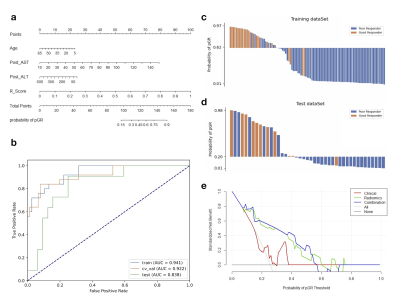
Osteosarcoma is the most common malignant osseous tumor and neoadjuvant chemotherapy for osteosarcoma has significantly improved survival outcomes. However, not all patients benefit from the current treatment strategy. We constructed a nomogram combined radiomics features from routinely available T2WI images and clinical variables to predict the response to neoadjuvant chemotherapy. The nomogram achieved an AUC of 0.838 (95% CI, 0.700-0.958) and DCA suggested that it has the potential to be used for preoperational prediction of pathological NAC response in osteosarcoma patients.
INTRODUCTION
Osteosarcoma is the most common malignant osseous tumor with two incidence peaks in children and adolescents, and in elders over 60 years 1. Nowadays, a conventional approach for osteosarcoma has significantly improved survival outcomes of osteosarcoma patients. However, not all patients benefit from the current treatment strategy. The purpose of our study was to develop and evaluate a radiomics model integrated the T2WI-based radiomics score and clinical variables to predict the response to neoadjuvant chemotherapy (NAC) in patients with osteosarcoma.METHODS
The workflow of this study is illustrated in Figure 1, which includes data inclusion and exclusion process and the radiomics workflow.Data: 144 osteosarcoma patients with average age of 17 years treated by NAC were included in this study. 36 (25.0%) patients were pathological good responders (pGR) to NAC, while 108 (75.0%) were non-pGR. They were split randomly into training (n = 101) and test (n = 43) datasets. The same datasets were used for the building and evaluation of all models, including clinical, radiomics, and combined model. Lesions were segmented on preoperative T2WI images by two radiologists independently.
Clinical Model: 21 clinical features with significant difference between pGR and non-pGR groups were selected using Relief algorithm, and 8 variables were retained in the final LR model.
Radiomics Model: We used open source software FeatureExplorer2 for feature extraction and model building. Shape, first order, and texture features were extracted from original images, Laplacian of Gaussian (LoG, sigma=1.0, 3.0, and 5.0) filtered and wavelet transformed images. Altogether, 702 features were extracted and used in radiomics model building. For feature selection, each classes of features from each type of the base image were used to build a scout model. The hyper-parameters of the scout models were tuned with 5-fold cross-validation using training dataset. The features retained in the scout models were used for the building of the final radiomics model.
For the modeling of the final radiomics model, dimension reduction was done with Pearson correlation coefficient after normalization. Feature selection was performed in the training dataset by 10-fold cross-validation after data balancing with upsampling.
Combined Model: The output radiomics score of the radiomics was merged with features retained in the clinical model to build the combined model.
Evaluation: Area under ROC curve (AUC), predicted probability plot, and decision curve analysis (DCA) were employed to evaluate the model performance and illustrate the nomogram utility. Confidence interval (CI) of AUCs were obtained by bootstrapping.
RESULTS
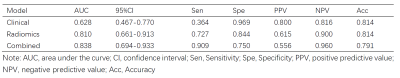
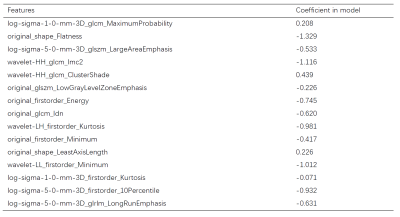
The results of the three models are listed in Table 1. The clinical and radiomics model achieved AUCs of 0.628 (95% confidence interval (CI): 0.404-0.857) and 0.810 (95% CI, 0.628-0.951) in the test dataset, respectively. Radiomics features contributing to the radiomics score are listed in Table 2, together with their corresponding weights. The clinical-radiomics nomogram combined radiomics score and clinical variables is shown in Figure 2, together with its ROC curve, prediction probability plot, and DCA curve. The nomogram demonstrated a good discrimination performance, with an AUC of 0.838 (95% CI, 0.700-0.958), better than the clinical or the radiomics model alone. The DCA suggested clinical utility of nomogram.DISCUSSION
The model combined radiomics score and clinical variables achieved the best performance in our study, which means radiomics features can be combined with clinical variables to predict neoadjuvant chemotherapy response more effectively. Our prediction models only relied on routinely collected raw care data, so they were more readily implementable in clinical environment.One shape feature representing tumor size was retained in the radiomics model, which has been demonstrated to be related to NAC response 3. Two kurtosis measures were found to be negatively correlated with probability of pGR, which might reflect the higher necrosis rate and lower heterogeneity of treated osteosarcoma, since higher kurtosis measures were thought to represent increased heterogeneity 4. The five texture features retained in the radiomics model also implied the importance of heterogeneity to the NAC response.
To our knowledge, this study was the first to utilize T2WI-based radiomics features and objective clinical variables to build a nomogram to predict NAC response of osteosarcoma patients preoperatively. There have been some radiomics studies in NAC response prediction with PET, CT, and advanced MRI sequences 5-8 previously. However, these images might not always be available in clinical settings. Delta-radiomics based on CT scans 5 require multiple time-point imaging data before and after NAC, which were hard to acquire for transferred patients. Contrast-enhanced MRI 6,7 presented excellent results, but they might not be accessible for fragile patients or for those with contrast agent allergy. PET showed certain predictive effects 8, but the cost of PET is high. Since MRI is an indispensable preoperational imaging procedure and our model only uses radiomics features from routine T2WI image, it will be much easier to be used in clinical settings.
CONCLUSION
The nomogram developed with radiomics based on conventional MRI sequence and objective clinical variables relied on routinely collected raw care data, has a potential of becoming a noninvasive, less human-dependent tool for preoperational prediction of pathological NAC response in osteosarcoma patients.Acknowledgements
No acknowledgement found.References
1. World Health Organization classification of tumors: WHO classification of tumours of soft tissue and bone, 5th edn. WHO Classification of Tumours Editorial Board, IARC Press, Lyon, 2020.
2. Song Y, Zhang J, Zhang Y, et al. FeAture Explorer (FAE): A tool of Model Development for Radiomics. Plos One. 2020; 15(8):e0237587.
3. Zhao S, Su Y, Duan J, et al. Radiomics signature extracted from diffusion-weighted magnetic resonance imaging predicts outcomes in osteosarcoma. J Bone Oncol. 2019;100263.
4. Davnall F, Yip CS, Ljungqvist G, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice. Insights Imaging. 2012; 3(6):573-589.
5. Lin P, Yang PF, Chen S, et al. A delta-radiomics model for preoperative evaluation of neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imaging. 2020; 20(1):7.
6. Kayal EB, Kandasamy D, Khare K, et al. Intravoxel incoherent motion (IVIM) for response assessment in patients with osteosarcoma undergoing neoadjuvant chemotherapy. Eur J Radiol 119 (2019): 108635.
7. Lee SK, Jee WH, Jung CK, et al. Prediction of poor responders to neoadjuvant chemotherapy in patients with osteosarcoma: additive value of diffusion-weighted MRI including volumetric analysis to standard MRI at 3T. PLoS One. 2020; 15(3):e0229983.
8. Song H, Jiao Y, Wei W, et al. Can pretreatment 18-F-FDG PET tumor texture features predict the outcomes of osteosarcoma treated by neoadjuvant chemotherapy, Eur Radiol. 2019; 29(7):3945–3954.
Figures