0006
Accelerated diffusion and relaxation-diffusion magnetic resonance imaging using time-division multiplexing echo-planar imaging (TDM-EPI)1Brigham and Women’s Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Boston Children’s Hospital, Boston, MA, United States
Synopsis
Recently, several studies have shown that brain tissue consists of microscopically heterogeneous components that are characterized by different T2 values and diffusivity. The joint relaxation-diffusion MRI technique has been developed to probe the intrinsic tissue microstructure that cannot be probed using standard dMRI. However, a major limitation of the relaxation-diffusion MRI technique is the long scan time for acquiring dMRI with multiple TEs. In order to significantly reduce the scan time, we propose a time-division multiplexing based echo-planar imaging (TDM-EPI) sequence, which can accelerate relaxation-diffusion MRI and standard dMRI by 2 or 3 folds.
Introduction
Joint relaxation-diffusion MRI techniques have been developed to probe the intrinsic tissue microstructure. Suitable joint analysis methods of this two-dimensional data has been used to develop several methods such as, diffusion-relaxation correlation spectroscopy1,2, relaxation-independent diffusion indices3 and T2-resolved multi-compartment models4-7 to improve tissue microstructural characterization. However, a major limitation of the joint relaxation-diffusion MRI technique is the long scan time required for dMRI with multiple echo times (TEs). To overcome these limitations, we propose an EPI-based pulse sequence using concepts of time-division multiplexing (TDM) referred as TDM-EPI to reduce the scan time. TDM-EPI interleaves excitation and readout train for two or more separate slices and exploits an echo-shifting technique8 to simultaneously acquire multiple slices. By arranging the sequence components for each slice and adjusting the echo-shifting gradients, the diffusion weighted images from separate slices can be acquired at same or different TE. Thus TDM-EPI will not only accelerate relaxation-diffusion MRI but also standard dMRI with a single TE. Based on experiments on phantom and in-vivo human brain imaging, we show that TDM-EPI can shorten scan time by two to three folds without reducing image quality when compared with the standard EPI.Methods
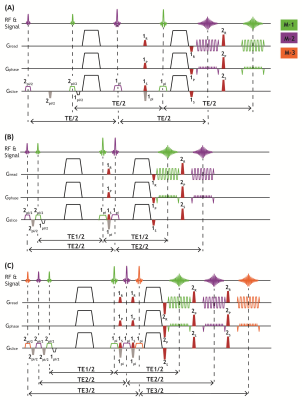
Figure 1shows the diagrams of three TDM-EPI sequences, referred to as TDM-2s, TDM-2e and TDM-3e, in which two or three sets of RF pulses are applied to two or three separate slices, with the corresponding event blocks being arranged so that TE can be the same or different across slices. Additional slice-rephasing gradients (gray color) are inserted to compensate for the phase dispersion induced by the slice-selection gradients. In order to separate the signals from different slices, an echo-shifting gradient (red color) is added between two 180° refocusing pulses to shift the k-space of one TDM slice away from that of the others. Before EPI readout of each TDM slice, a rephasing gradient is added to rephase the echo signal of one slice and concurrently dephase that of the other slices. We specified the amplitude of echo-shifting gradient as $$$G_{x}(t)=G(t)cos\theta$$$, $$$G_{y}(t)=G(t)sin\theta$$$ in the sequence implementation to keep the amplitude as G(t). We introduce an echo shifting factor $$$k_{shift}=\gamma\sqrt{(\int_{}^{}G_{x}(t)dt)^{2}+(\int_{}^{}G_{y}(t)dt)^{2}}=\gamma\int_{}G(t)dt$$$ to represent the shifting degree. To determine the optimal echo-shifting gradient, we evaluated the relationship between signal leakage level across from one slice to the other and the shifting factor by only turning on one excitation RF pulse. The echo-shifting gradients have minor contributions to diffusion-encoding. Though the b-value of the echo-shifting gradient alone is negligible, e.g. ~2 s/mm2, it may be coupled with the diffusion gradient and lead to non-negligible double diffusion encoding (DDE) effect that depends on the relative angle between the two. To eliminate the coupling between the two gradients, we adaptively applied the echo-shifting gradient along the orthogonal direction to the diffusion gradient, thereby nearly eliminating this effect. Data were obtained on a 3T MAGNETOM Prisma scanner using the proposed TDM-EPI and standard EPI sequences. We acquired diffusion image datasets with a single TE and multiple TEs using TD-2s and TDM-2e, TDM-3e sequences, respectively. The scan protocols for the diffusion experiments were as follows: TR =3000ms, FOVxy=210×210mm2, PF=6/8, 2.5mm isotropic resolution, TE =78 for TDM-2s, two sets of TE (70 ms, 110 ms) and (90 ms, 135 ms) for TDM-2e, TE = (72 ms, 102 ms and 132 ms) for TDM-3e. Datasets with same parameters were collected for comparison using standard EPI.Results
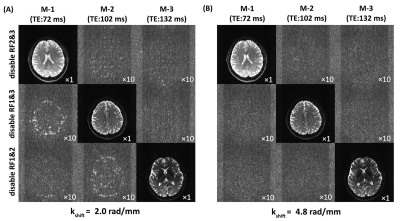
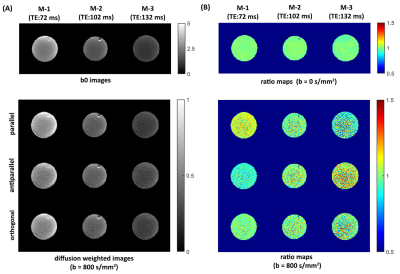
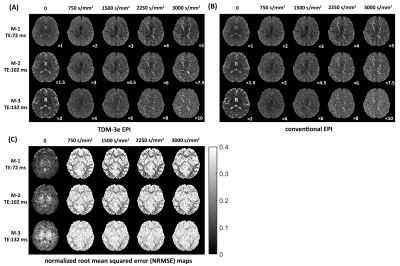
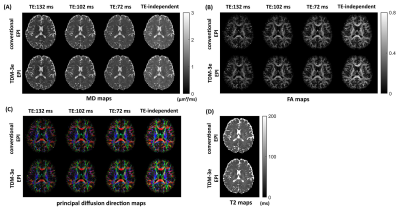
To show that suitable echo-shifting gradients can minimize the signal leakage across slices, Figs. 2A and 2B illustrate the three slices from a TDM-3e sequence with $$$k_{shift}$$$=2.0 rad/mm and $$$k_{shift}$$$=4.8 rad/mm. Residual signals can be observed with $$$k_{shift}$$$=2.0 rad/mm while can hardly be noticed with $$$k_{shift}$$$=4.8 rad/mm. To examine the coupling between the echo-shifting and diffusion gradients, three representative directions of echo-shifting gradient relative to diffusion gradients have been investigated on water phantom. Figure 3 shows the phantom images obtained by TDM-3e and the corresponding ratio maps of the images to those obtained by standard EPI. For b= 800 s/mm2, a significant difference between three ratio maps can be observed in parallel and antiparallel directions. On the other hand, the signal intensities are barely different when the two gradients are in orthogonal directions. Figures 4 compares the images obtain by TDM-3e and conventional EPI sequences. Figures 5A-5C illustrate the MD, FA and the color-coded DTI maps with different TEs of one slice obtained by the TDM-3e and conventional EPI sequences, respectively. The T2 maps and the TE-independent DTI-based metrics estimated using REDIM9 are obtained by joint analysis of acquired data with multiple TE.Discussion and Conclusion
We proposed a set of TDM-EPI pulse sequences that can accelerate the acquisition of dMRI with a single or multiple TE by a factor of 2x or 3x. We optimized the echo-shifting gradients using a water phantom and using in-vivo human brain scans and validated that the acquired images using TDM-EPI had similar quality as those acquired by conventional EPI. Thus, TDM-EPI can potentially accelerate scan time of standard dMRI and relaxation-diffusion MRI to allow its widespread use to investigate mental disorders.Acknowledgements
This study was supported in part by NIH grants R21MH116352, K01MH117346, R01MH119222, R01MH116173.References
1. Hürlimann M, Venkataramanan L. Quantitative measurement of two-dimensional distribution functions of diffusion and relaxation in grossly inhomogeneous fields. J Magn Reson 2002;157(1):31-42.
2. Kim D, Doyle EK, Wisnowski JL, Kim JH, Haldar JP. Diffusion‐relaxation correlation spectroscopic imaging: a multidimensional approach for probing microstructure. Magn Reson Med 2017;78(6):2236-2249.
3. Ning L, Setsompop K, Westin CF, Rathi Y. New insights about time‐varying diffusivity and its estimation from diffusion MRI. Magn Reson Med 2017;78(2):763-774.
4. Veraart J, Novikov DS, Fieremans E. TE dependent Diffusion Imaging (TEdDI) distinguishes between compartmental T2 relaxation times. Neuroimage 2018;182:360-369.
5. Gong T, Tong Q, He H, Sun Y, Zhong J, Zhang H. MTE-NODDI: Multi-TE NODDI for disentangling non-T2-weighted signal fractions from compartment-specific T2 relaxation times. Neuroimage 2020:116906.
6. Tax CM, Szczepankiewicz F, Nilsson M, Jones DK. The dot-compartment revealed? Diffusion MRI with ultra-strong gradients and spherical tensor encoding in the living human brain. Neuroimage 2020;210:116534.
7. Lampinen B, Szczepankiewicz F, Mårtensson J, van Westen D, Hansson O, Westin CF, Nilsson M. Towards unconstrained compartment modeling in white matter using diffusion‐relaxation MRI with tensor‐valued diffusion encoding. Magn Reson Med 2020;84(3):1605-1623.
8. Bishop JE, Plewes DB. TE interleaving: new multisection imaging technique. J Magn Reson Imaging 1991;1(5):531-538.
9. Ning L, Gagoski B, Szczepankiewicz F, Westin C-F, Rathi Y. Joint RElaxation-Diffusion Imaging Moments to Probe Neurite Microstructure. IEEE Trans Med Imaging 2019;39(3):668-677.
Figures