0005
Interleaved MRI and DMI on human brain in vivo
Yanning Liu1, Henk M. De Feyter1, Scott McIntyre1, Terence W. Nixon1, and Robin A. de Graaf1
1MRRC Yale University, New Haven, CT, United States
1MRRC Yale University, New Haven, CT, United States
Synopsis
Deuterium metabolic imaging (DMI) is a powerful method to map metabolism in vivo. To integrate DMI with clinical MRI, we propose and demonstrate an interleaved MRI and DMI routine, including the necessary hardware and sequence modifications. Using interleaved FLAIR MRI+DMI as an example, we demonstrate that MR image quality and DMI sensitivity as well as information content are preserved, both in phantoms and in the human brain in vivo. The interleaved MRI+DMI technology provides full flexibility to extend any MRI protocol with DMI, thereby offering a metabolic component to the range of MR imaging contrasts.
Introduction
Deuterium metabolic imaging (DMI) is a robust and simple MR-based method to map metabolic information in vivo. It has been successfully used to evaluate metabolism in various tissues and to characterize glioblastoma multiforme (GBM) brain tumours in patients. 1-4 Although the potential benefits of supplementing clinical MRI with DMI are numerous, the sequential acquisition of standard MRI and DMI may be too time-consuming and compromise patient compliance. To overcome this restriction, we propose an interleaved MRI+DMI routine, that capitalizes on the fact that 1H-based MRI and 2H-based DMI are independent modalities that can be acquired in parallel. As most scanners can only acquire signal at a single Larmor frequency (e.g. 1H), additional hardware is required to convert the 2H DMI signal into a 1H frequency. Using interleaved FLAIR MRI+DMI as a demonstration, we show that, by strategically incorporating 2H pulse-acquisition segments into the inherent waiting periods of an 1H MRI sequence, MRI and DMI can be acquired in parallel without an increase in scan time. Here we illustrate the performance of MRI+DMI in both phantom and human brain in vivo.Methods
Hardware: All data were acquired on a Bruker 4T 94cm Medspec system (Bruker Corporation. Ettlingen, Germany) using a single channel TEM 1H volume coil and a 4 channel phased array 2H coil. To achieve successive 1H – 2H signal reception, the 2H signal was up-converted to the 1H frequency before detection via a custom-built up-conversion unit (Figure 1). The unit consists of an RF mixer plus an RF switch and a Direct Digital Synthesizer (DDS) Local Oscillator (LO). Directly controlled by the scanner pulse program, it mixes the native 26.2 MHz 2H signal with 144.3 MHz to generate a 170.5 MHz 2H signal that is detected as a standard 1H signal. Interleaved FLAIR MRI+DMI sequence: Interleaved FLAIR MRI+DMI data was acquired using the scheme shown in Figure 2. A standard FLAIR sequence (TR/TI/TE = 8800/2200/90 ms, 256 x 192 matrix over 256 x 192 mm2, 14 slices of 3 mm) was interleaved with DMI by utilizing the unused delays inherent to the FLAIR MRI method. With an acquisition time of 40 ms and a TR of 314 ms, a total of 28 DMI acquisitions could be interleaved during each FLAIR MRI repetition. DMI was acquired as a 13 x 9 x 11 matrix (spherical k-space encoding) over 260 × 180 × 220 mm3. For a total FLAIR MRI acquisition time of 7 minutes, 2 complete DMI datasets were acquired. In vivo study: Interleaved FLAIR MRI+DMI data were acquired in a healthy volunteer, 60-75 min after oral intake of 55 g of [6,6’-2H2]-glucose dissolved in 250 mL of water, as described previously2.Results
Figure 3 compares 2H MR signals and spectra acquired with the (A, C) direct and the (B, D) interleaved method on human brain in vivo after administration of [6,6-2H2]-glucose. Both spectra exhibit a linear phase roll due to delayed acquisition, whereas the interleaved FID is also truncated due to the shortened acquisition time. Artifacts due to delayed acquisition and truncation can be removed through singular value decomposition (SVD)-based extrapolation. The direct (SNR=418) and interleaved (SNR=421) SNR values are comparable, demonstrating that interleaved acquisition can be performed without sensitivity penalty.The FLAIR MRI and DMI acquired using (A) direct DMI and (B) interleaved FLAIR MRI+DMI in a phantom containing deuterated water and DMSO is shown in Figure 4. Both DMI data are overlaid on the interleaved FLAIR MRI. The information content, as well as the SNR and spectral resolution between the direct and interleaved DMI data is equivalent, further underlining that MRI and DMI can be acquired in parallel without reducing quality. Figure 5. shows interleaved FLAIR-DMI obtained from human brain in vivo following the administration of deuterated glucose. The DMI data represents one axial (13 x 9) plane extracted from a 3D (13 x 9 x 11) dataset, whereas the FLAIR MRI represents one axial slice from a 14-slice dataset. The DMI data displays the familiar spectral fingerprint of water, glucose and glutamate+glutamine as reported previously2. It should be noted that the DMI only represents two signal averages, acquired in 7 min. The FLAIR MR images are characterized by minimal image contrast and elimination of ventricular water signal, as expected for healthy human brain.Discussion
Interleaved and simultaneous acquisition of MRI and an X-nucleus-based MR method is an excellent strategy to reduce scan time without sacrificing data quality. 5-7 Interleaved MRI+DMI is attractive for clinical applications as it essentially doubles the information content of an MRI study by obtaining both anatomical data and information on active metabolism. In this study, we successfully applied interleaved FLAIR MRI+DMI on a phantom, and in human brain in vivo. Compared to the direct 2H experiments, the interleaved measurements are accurate without loss of sensitivity or the introduction of artifacts. The interleaved MRI+DMI acquisition strategy can be extended to a wide range of clinically relevant MRI methods, including T1, T2, susceptibility and diffusion-weighted sequences. Whereas each MRI method will have its own unique demands on 2H pulse and acquisition placement, the presented methodology is sufficiently flexible to accommodate most, if not all, MRI methods.Acknowledgements
This research was funded, in part, by NIH grant NIBIB R01-EB025840.References
1. Lu, M.; Zhu, X.-H.; Zhang, Y.; Mateescu, G.; Chen, W., Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Journal of Cerebral Blood Flow & Metabolism 2017, 37 (11), 3518-3530.2. De Feyter, H. M.; Behar, K. L.; Corbin, Z. A.; Fulbright, R. K.; Brown, P. B.; McIntyre, S.; Nixon, T. W.; Rothman, D. L.; de Graaf, R. A., Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Science Advances 2018, 4 (8), eaat7314.3. Kreis, F.; Wright, A. J.; Hesse, F.; Fala, M.; Hu, D.-e.; Brindle, K. M., Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 2019, 294 (2), 289-296.4. Riis-Vestergaard, M. J.; Laustsen, C.; Mariager, C. Ø.; Schulte, R. F.; Pedersen, S. B.; Richelsen, B., Glucose metabolism in brown adipose tissue determined by deuterium metabolic imaging in rats. International Journal of Obesity 2020.5. Friedman, S. D.; Jensen, J. E.; Frederick, B. B.; Artru, A. A.; Renshaw, P. F.; Dager, S. R., Brain changes to hypocapnia using rapidly interleaved phosphorus-proton magnetic resonance spectroscopy at 4 T. J Cereb Blood Flow Metab 2007, 27 (3), 646-53.6. Meyerspeer, M.; Magill, A. W.; Kuehne, A.; Gruetter, R.; Moser, E.; Schmid, A. I., Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magnetic Resonance in Medicine 2016, 76 (5), 1636-1641.7. Yu, Z.; Madelin, G.; Sodickson, D. K.; Cloos, M. A., Simultaneous proton magnetic resonance fingerprinting and sodium MRI. Magnetic Resonance in Medicine 2020, 83 (6), 2232-2242.Figures
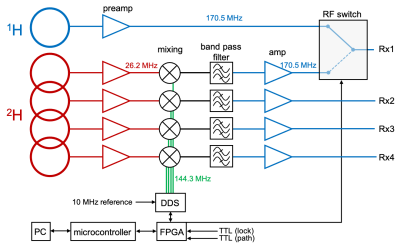
Configuration of the up-conversion unit. The 1H signal is sent to one arm of the RF switch. The 2H signal is supplied to an RF mixer and up-converted to 170.5 MHz by mixing with the 144.3 MHz Direct Digital Synthesizer (DDS) Local Oscillator (LO). This signal is filtered, amplified and sent to the RF switch. During the acquisition, the RF switch, and the phase and frequency of DDS are controlled in real time through a field-programmable gate array (FPGA) via TTL triggers and a network PC.
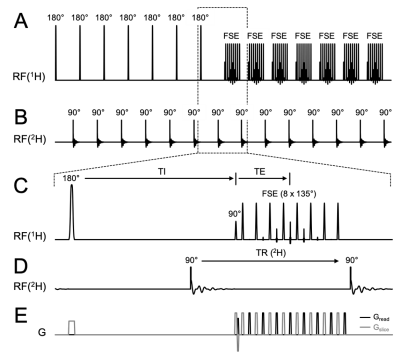
Interleaved FLAIR MRI+DMI sequence. (A) 1H pulse acquisition scheme. 7 slices are inverted consecutively followed by a fast spin-echo (FSE) acquisition (TR/TI/TE = 8800/2200/90 ms). The (B) 2H pulse acquisitions are placed in the dead time during TI and right after FSE with equal TR (= 314 ms). (E) Shows the gradients used during acquisition. Note the plot only shows the first half of FLAIR TR, where half of the slices (odd number slices) were excited. The pulse acquisition scheme is repeated on the even number slices in the second half of TR. 28 DMI acquisitions are achieved in one FLAIR TR.
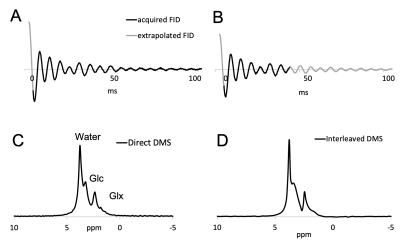
(A, C) Direct and (B, D) interleaved 2H FID signals and spectra acquired from human brain acquired 60 min following oral [6,6’-2H2]-glucose administration. The (A) direct FID was extrapolated backwards using singular value decomposition (SVD) to the timepoint right after excitation. Using SVD, the (B) indirect FID was extrapolated backwards and forwards to match the time base of the direct FID. Note that data extrapolation is only a cosmetic operation that improves the visualization of MR spectra. It is not necessary when signals are quantified in the time domain.
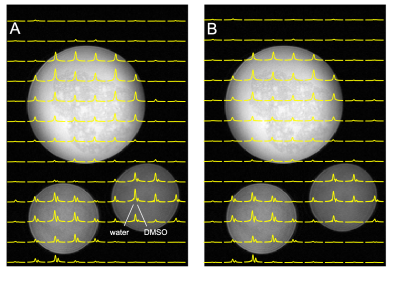
(A) Direct DMI and (B) Interleaved DMI and FLAIR MRI of a phantom containing ~0.1% D2O and various amounts of DMSO-D6 (~0.02 – 0.05%) acquired as a 13 x 9 x 11 matrix over a 260 x 180 x 220 mm3 FOV.
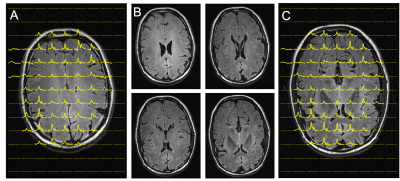
Interleaved FLAIR and DMI of human brain 75 min following the oral administration of [6,6’-2H2]-glucose. DMI was acquired as a 13 x 9 x 11 matrix (YXZ) over a 260 x 180 x 220 mm3 FOV, whereas the FLAIR images were acquired as 14 slices and a 256 x 192 matrix over a 256 x 192 mm2 FOV (TR/TI/TE = 8800/2200/90 ms). During the ~ 7 min FLAIR acquisition, the DMI data could be acquired twice (i.e. NA = 2). (A-C) FLAIR images spanning circa 40 mm, covering two axial planes from the 3D DMI dataset as shown in (A) and (C).