0003
Maxwell Compensation for Spiral Turbo-Spin-Echo Imaging1University of Virginia, Charlottesville, VA, United States, 2Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Spiral TSE imaging presents challenges for compensating concomitant (Maxwell) gradient effects because spiral waveforms vary along the echo train, as opposed to Cartesian imaging for which the same readout waveform is used for every echo. Since Maxwell terms are proportional to 1/Bo, compensation is particularly important at low field strength. An interleaved-spiral T2-weighted 2D-TSE pulse sequence was developed that incorporates gradient waveform modifications to achieve compensation of the self-squared Maxwell terms at both the echoes and over echo spacings. This approach provided substantial improvement in image quality at 0.55T for degradation associated with self-squared concomitant-gradient effects.
Introduction & Purpose:
Compared to widely used Cartesian sampling, spiral trajectories offer advantages for acquisition speed, SNR efficiency, and robust performance with motion. Recently, promising results for interleaved-spiral T2-weighted 2D turbo-spin-echo (TSE) imaging were demonstrated by Li et al.1. Concomitant (Maxwell) fields are a potential source of artifacts in TSE imaging2. Although concomitant-gradient effects in Cartesian-TSE imaging have been well described2, spiral TSE imaging presents additional challenges because spiral waveforms vary along the echo train, as opposed to Cartesian imaging for which the same readout waveform is used for every echo.A potentially important application area for spiral TSE is low-field imaging, since off-resonance effects decrease as field strength decreases. Interesting results at low field have recently been shown using a high-performance MR scanner at 0.55T3. Although off-resonance effects are reduced at lower field strengths, Maxwell terms are proportional to 1/Bo, and thus concomitant-gradient effects increase as field decreases.
At 3T, using spiral in-out gradient waveforms (which have inherent symmetry with respect to the echo), Li et al. found that good image quality was obtained by adding only a compensation gradient during the readout prephaser period, which balanced the concomitant self-squared terms at the first echo1. However, this approach is likely insufficient at much lower magnetic field strengths and would not perform well for spiral trajectory types that are not symmetric, such as spiral out. The purpose of this work was to implement interleaved-spiral T2-weighted 2D-TSE imaging with compensation for self-squared concomitant-gradient terms at every echo, and across every echo spacing, along the echo train. Since trajectory angles, and thus spiral waveforms, vary along the echo train, this approach requires gradient-waveform modifications along the entire echo train.
Methods:
A prototype 2D-TSE pulse sequence was developed to support interleaved-spiral acquisition using spiral-out, spiral-in or spiral-in-out trajectories. The goal of the Maxwell-compensation algorithm was to reduce the phase shift from self-squared terms at each echo (target phase shift of zero), and reduce the difference in phase shifts among echo spacings (target of constant phase shift at the end of each echo spacing). This was accomplished by:1. Modifying trapezoidal gradient segments applied during each echo spacing. Lengthening (or shortening) trapezoidal gradient segments of the original waveform permits the Maxwell integral to be decreased (or increased), while maintaining the original zeroth gradient moment.
2. Adding zero-moment waveforms (bipolar gradients for this preliminary implementation). Adding bipolar waveforms permits the Maxwell integral to be further increased, as needed, while also maintaining the original zeroth gradient moment.
The same procedure was used independent of trajectory type (out, in, or in-out).
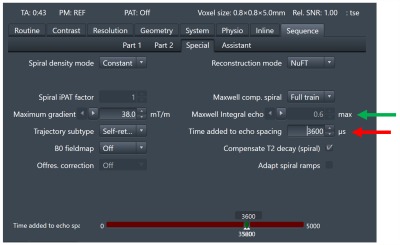
In general, additional time must be added to the echo spacing to achieve Maxwell compensation. For this preliminary investigation, the pulse-sequence user interface was modified to permit the user to choose the additional time to add for each echo spacing. The pulse sequence then performs compensation within the chosen time constraint and reports the maximum Maxwell integral remaining among echoes and the maximum difference in integral values among echo spacings. This implementation adapts to changes in pulse sequence parameters and spiral design to minimize the Maxwell integrals. The user interface is illustrated in Figure 1.
The spiral 2D TSE with Maxwell compensation was tested in phantoms and healthy volunteers (after obtaining informed consent) on 1.5T (MAGNETOM Avanto) and 0.55T (prototype MAGNETOM system) MR scanners (both Siemens Healthcare, Erlangen, Germany).
Results & Discussion:
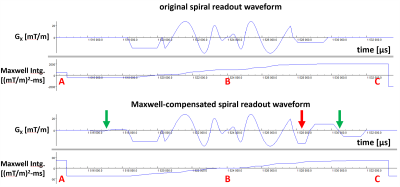
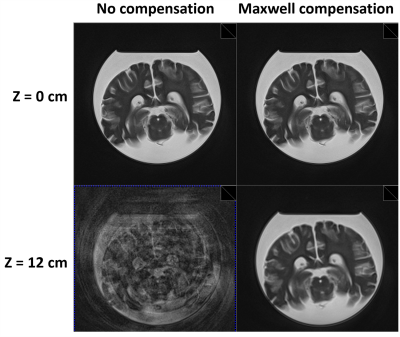
Figure 2 illustrates modifications made to a representative spiral-in-out gradient waveform to achieve full Maxwell compensation along the echo train. The red arrows indicate trapezoidal gradients that were modified (compared to no compensation), and the green arrows indicate bipolar gradients that were added, to achieve compensation. After compensation, the Maxwell integral at the end of the echo spacing (labeled C) is equal to that at the end of the preceding echo spacing (labeled A), and the Maxwell integral at the echo is zero (labeled B).Figure 3 shows 2D spiral-out TSE axial images of an ex vivo brain (1.5T), illustrating the effectiveness of the proposed Maxwell-compensation procedure. While both uncompensated and compensated images show no significant artifacts at slice position z = 0 cm (upper row), where additional phase from concomitant gradients is expected to be zero, the uncompensated image shows substantial artifacts at slice position z = 12 cm (lower row, left). With concomitant-gradient compensation, no significant artifacts are seen at z = 12 cm (lower row, right).
Figure 4 illustrates the image quality obtained in vivo for T2-weighted brain imaging at 0.55T (see caption for pulse-sequence parameters).
Conclusions & Future Work:
We demonstrated an interleaved-spiral T2-weighted 2D-TSE pulse sequence that incorporates gradient waveform modifications to achieve compensation of self-squared Maxwell terms at both the echoes and over echo spacings. This approach provided substantial improvement in image quality for degradation associated with self-squared concomitant-gradient effects. Further studies are needed to determine if concomitant-gradient cross-terms4 will also require compensation to achieve high image quality for a wide variety of clinically relevant imaging scenarios, especially at low field strengths. Future studies will explore optimization of the algorithm to decrease the time added in each echo spacing compared to that required for the current algorithm, which will reduce the sacrifice in acquisition efficiency for achieving Maxwell compensation.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare.References
1. Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magn Reson Med 2018; 80:1989-1996.
2. Zhou XJ, Tan SG, Bernstein MA. Artifacts induced by concomitant magnetic field in fast spin-echo imaging. Magn Reson Med 1998; 40:582-591.
3. Campbell-Washburn AE et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019; 293:384-393.
4. Bernstein MA et al. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med 1998; 39:300-308.
Figures