0002
VUDU: motion-robust, distortion-free multi-shot EPI1Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 4Radiological Sciences Laboratory, Stanford University, Palo Alto, CA, United States
Synopsis
We introduce VUDU (Variable flip, blip-Up and -Down Undersampling) for motion-robust, distortion-free multi-shot EPI (msEPI) acquisition. VUDU uses FLEET-ordering to acquire all shots of a given slice successively before proceeding to the next slice, and employs variable flip angle (vfa) excitation to maximize the signal. Phase encoding polarities are reversed between shots to estimate and eliminate distortions, and low-rank constraint mitigates shot-to-shot inconsistencies. VUDU thus utilizes vfa-FLEET excitation and blip-up and -down acquisition (BUDA) to encode each slice in 250ms. We demonstrate VUDU with GRE, SE/diffusion contrasts in the brain, and expect that this will enable msEPI in the abdomen.
Introduction
msEPI acquisition can mitigate distortion, T2-/T2*-related voxel blurring and voxel pile-ups. These are made possible by increasing the in-plane acceleration, Rinplane, and combining the shots together to alleviate noise amplification. However, the several seconds required to acquire multiple shots increase vulnerability to motion and shot-to-shot phase variations. These variations can be mitigated using navigators (1) or constrained reconstruction (2–5). Rigid motion models can also be incorporated into the reconstruction (6,7). Despite these advances, extending msEPI to abdominal or cardiac imaging requires alternate acquisition strategies.FLEET-ordered msEPI (8) acquires all shots for a specific slice first, then proceeds to the next slice. This reduces the acquisition timeframe for each slice and boosts motion robustness. FLEET has been used as a calibration scan (8), and as the primary imaging acquisition (9,10). As the short slice repetition time (TRslice) does not permit significant T1 recovery, variable flip angles (vfa) of $$$\theta_1=45^{\circ}$$$ and $$$\theta_2=90^{\circ}$$$ are utilized to maximize the signal.
We extend vfa-FLEET to GRE, SE and diffusion contrasts. We alternate the phase encoding polarities between the two-shots and perform blip -up/-down acquisitions (BUDA) (11,12), rely on low-rank constraint to mitigate shot-to-shot variations, and incorporate a fieldmap into the reconstruction to eliminate distortion (13). Combined, we perform vfa-FLEET excitation and BUDA reconstruction. We term this technique VUDU (Variable flip, blip-Up and -Down Undersampling).
Data/code: https://bit.ly/3oYPetX
Pulse Sequence
(Fig1, top) shows the VUDU sequence for GRE contrast. A particular slice is excited by a $$$\theta_1=45^{\circ}$$$ pulse and encoded by a blip-up readout. Immediately after, a second excitation at $$$\theta_2=90^{\circ}$$$ is encoded with a blip-down readout. SNR of vfa-FLEET is proportional to $$$sin\theta_1$$$, which is reduced by ~30% relative to standard msEPI ordering.Image Reconstruction
Separate SENSE (14) reconstructions for each shot (Fig1-bottom) were processed with topup (15,16) to estimate a fieldmap, $$$E$$$, which was used to jointly reconstruct the two shots:$$min_x \sum_{t=1}^2 \parallel F_t E C x_t - d_t \parallel _2^2 +\lambda\parallel H(\bf x)\parallel_* (Eq1)$$
where $$$C$$$ are sensitivities (from a 4sec prescan using ESPIRiT (17)), $$$t=1$$$ stands for blip-up, and $$$t=2$$$ for -down shot, $$$F_t$$$ is the undersampled Fourier operator in $$$t$$$th shot, $$$x_t$$$ is the distortion-free image and $$$d_t$$$ are the k-space data for shot $$$t$$$. The constraint $$$\parallel H(\bf x)\parallel_*$$$ enforces Hankel low-rank prior on the two-shot data $$$\bf x$$$ in k-space.
GRE Acquisition
vfa-FLEET GRE data were acquired using Rinplane=3 with ∆ky=1 shift between the shots to provide complementary information. Matrix size was 148×148×32 at 1.5×1.5×4mm3 resolution using TE=36msec (minimum possible) on a Siemens Skyra equipped with a 32-chan coil.Fig2: shows SENSE for each shot and the distortion-free VUDU reconstruction. Arrows point to distortion artifacts in sagittal views of SENSE reconstructions, which were addressed in VUDU.
SENSE images from each shot had similar signal levels, with $$$ \parallel x_{up}^{sense} \parallel_2 / \parallel x_{down}^{sense} \parallel_2=0.93$$$. This scaling factor was incorporated into VUDU reconstruction.
SE and Diffusion Acquisition
msEPI with standard slice-segment ordering and vfa-FLEET with matching resolution (1.5×1.5×4 mm3) were acquired using Rinplane=3, TE=64msec on a Siemens Prisma system and a 32-chan coil.Fig3a: shows b=0 images from standard msEPI, where BUDA was able to eliminate distortion by jointly reconstructing the shots, as indicated by horizontal lines.
Fig3b: presents individual SENSE and joint VUDU reconstructions for the vfa-FLEET acquisition. VUDU was successful in eliminating distortion, and at TRslice=113msec, it took 226msec to encode each slice.
Both standard and vfa-FLEET acquisitions took 7.2sec for the entire brain.
To highlight the difference in signal level, standard and vfa-FLEET were windowed identically. The signal ratio $$$ \parallel x_{vudu} \parallel_2 / \parallel x_{buda} \parallel_2=0.78$$$ was slightly higher than the theoretical $$$sin45^{\circ}=0.71$$$.
Arrows point to regions of low signal in the blip-down vfa-FLEET shot. The ratio of the two shots $$$ \parallel x_{up}^{sense} \parallel_2 / \parallel x_{down}^{sense} \parallel_2=1.29$$$ was used to compensate signal differences during VUDU reconstruction.
Fig4: shows 6-direction vfa-FLEET diffusion data with VUDU reconstruction at b=1000s/mm2. Estimating a fieldmap per direction corrected bulk B0 and eddy current distortions, providing high geometric consistency.
Fig5 shows average diffusion images for the vfa-FLEET acquisition. VUDU is able to provide high geometric fidelity in these lower slices. Low signal regions are present in the blip-down shot (arrows), which in part propagated to VUDU reconstruction.
Discussion
We presented VUDU for motion-robust, distortion-free msEPI. We anticipate GRE-VUDU to be impactful in high resolution fMRI, and SE/diffusion-VUDU to find applications in neuroimaging of motion-prone populations. A tradeoff made for the motion-robustness is the SNR reduction compared to standard slice ordering, which was 22% in the current diffusion data.We think that incorporating spoilers after the 1st readout will mitigate the low signal regions in the 2nd shot in SE/diffusion experiments. Signal levels from the two-shots were similar in GRE acquisition, where there is a smaller time window between the shots. In SE/diffusion, inversion of Mz by the first refocusing pulse leads to accelerated T1 recovery during the inter-shot gap (18). This may, in part, explain the signal level differences. Advanced pulse design can reduce differences in slice profiles between the vfa shots to address the remaining variation (9,10,18). With these improvements, VUDU can finally bring benefits of msEPI acquisition to fetal, cardiac and abdominal imaging, where motion is unpredictable and non-rigid.
Acknowledgements
This work was supported by research grants NIH R01 EB028797, U01 EB025162, P41 EB030006, U01 EB026996 and the NVidia Corporation for computing support.References
1. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn. Reson. Med. 2009;62:468–475. doi: 10.1002/mrm.22024.
2. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41–47. doi: 10.1016/J.NEUROIMAGE.2013.01.038.
3. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS). Magn. Reson. Med. 2017;78:494–507. doi: 10.1002/mrm.26382.
4. Haldar JP. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans. Med. Imaging 2014;33:668–681. doi: 10.1109/TMI.2013.2293974.
5. Lee J, Jin KH, Ye JC. Reference-free single-pass EPI Nyquist ghost correction using annihilating filter-based low rank Hankel matrix (ALOHA). Magn. Reson. Med. 2016;76:1775–1789. doi: 10.1002/mrm.26077.
6. Guhaniyogi S, Chu M, Chang H, Song AW, Chen N. Motion Immune Diffusion Imaging Using Augmented MUSE for High-Resolution Multi-Shot EPI. 2016;652:639–652. doi: 10.1002/mrm.25624.
7. Wang F, Bilgic B, Dong Z, et al. Motion-robust sub-millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magn. Reson. Med. 2018;80:1891–1906. doi: 10.1002/mrm.27196.
8. Polimeni JR, Bhat H, Witzel T, Benner T, Feiweier T, Inati SJ, Renvall V, Heberlein K, Wald LL. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn. Reson. Med. 2016;75:665–679. doi: 10.1002/mrm.25628.
9. Berman AJ, Witzel T, Grissom WA, Park D, Setsompop K, Polimeni JR. High-resolution segmented-accelerated EPI using Variable Flip Angle FLEET with tailored slice profiles. In: Proc Intl Soc Mag Reson Med. ; 2019. p. 1169.
10. Berman AJL, Grissom WA, Witzel T, Nasr S, Park DJ, Setsompop K, Polimeni JR. Ultra‐high spatial resolution BOLD fMRI in humans using combined segmented‐accelerated VFA‐FLEET with a recursive RF pulse design. Magn. Reson. Med. 2021;85:120–139. doi: 10.1002/mrm.28415.
11. Liao C, Cao X, Cho J, Zhang Z, Setsompop K, Bilgic B. Highly efficient MRI through multi-shot echo planar imaging. 2019;1113818:43. doi: 10.1117/12.2527183.
12. Cao X, Liao C, Zhang Z, Iyer SS, Wang K, He H, Liu H, Setsompop K, Zhong J, Bilgic B. T2-BUDA-gSlider: rapid high isotropic resolution T2 mapping with blip-up/down acquisition, generalized SLIce Dithered Enhanced Resolution and subspace reconstruction. ArXiv 2019;1909.12999.
13. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. Neuroimage 2017;153:97–108. doi: 10.1016/J.NEUROIMAGE.2017.03.052.
14. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999;42:952–962.
15. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7.
16. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051.
17. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014;71:990–1001.
18. Berman A, Grissom W, Witzel T, Park D, Veissmann O, Setsompop K, Polimeni J. Segmented spin-echo BOLD fMRI using a variable flip angle FLEET acquisition with recursive RF pulse design for high spatial resolution fMRI. In: Proc Intl Soc Mag Reson Med. ; 2020. p. 3881.
Figures
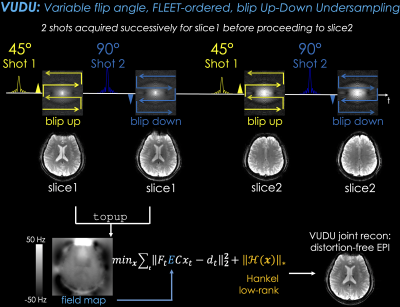
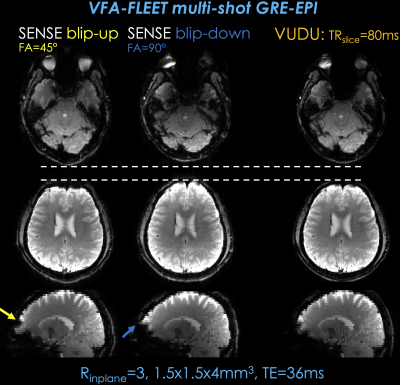
Fig2. shows individual SENSE reconstructions for each shot of vfa-FLEET acquisition with GRE contrast. At TRslice=80 msec, it took vfa-FLEET 160 msec to encode each slice, and 5.1 sec for the entire volume.
Horizontal lines help compare the geometric fidelity of distortion-free VUDU reconstruction relative to each shot. Yellow and blue arrows point to distortion artifacts in the sagittal views of SENSE reconstructions, which were eliminated in the VUDU result.
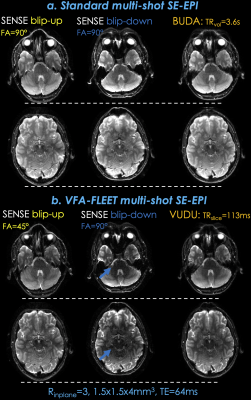
Fig3a. shows b=0 images from a standard slice-ordered msEPI acquisition, where BUDA was able to eliminate distortion by jointly reconstructing the two shots, as indicated by the horizontal dashed lines.
Fig3b. presents individual SENSE and joint VUDU reconstructions for the vfa-FLEET acquisition. VUDU successfully eliminated distortion, and encoded each slice in 226 msec.
Standard and vfa-FLEET images are windowed identically. VUDU / BUDA signal ratio was 78%, and was slightly higher than theoretical 1/sin45°. Arrows point to low signal regions in the blip-down vfa-FLEET shot.
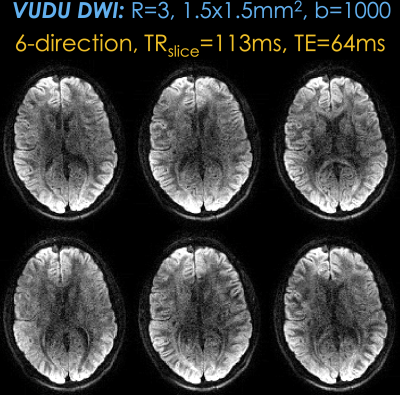
Fig4. 6-direction vfa-FLEET diffusion acquisition with VUDU reconstruction at b=1000 s/mm2. With TRslice = 113 ms, acquisition of each slice was completed in 226 ms to provide motion robustness. Estimating the field map per each direction allowed VUDU to correct both the bulk B0 and eddy current distortions, providing high geometric consistency between the directions.
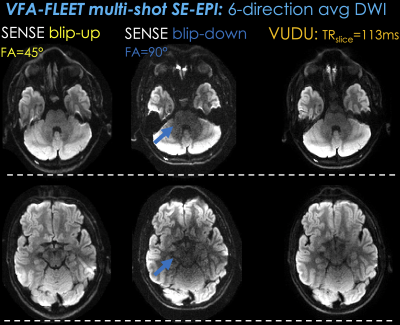
Fig5. shows average diffusion images across the 6 directions for the vfa-FLEET acquisition. Individual SENSE reconstructions for the blip-up and -down shots exhibit distortions, whereas VUDU is able to provide high geometric fidelity even in these lower slices with poor field homogeneity. Low signal regions are present in the blip-down shot (blue arrows), which have in part propagated to the VUDU reconstruction, and will likely be mitigated with gradient spoiling.