0001
Magnetic Resonance Coherence Pathway Unraveling
Nikolai Mickevicius1 and Eric Paulson2
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI, United States
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
The magnetic resonance coherence pathway unraveling (MR-CPU) method acquires primary and stimulated echoes simultaneously, and encodes them using CAIPIRINHA RF phase cycling such that they can be separated during image reconstruction. This initial study demonstrates the feasibility of unaliasing overlapped coherence pathway images, and future studies will investigate its use for quantitative T1, T2, and diffusion coefficient mapping.
Introduction
Here we present a novel approach termed magnetic resonance coherence pathway unraveling (MR-CPU) to disentangle overlapped spin echoes originating from different coherence pathways to produce multiple images with unique, diagnostic quality contrast from a single acquisition. This capability is attained by means of RF phase modulation over measured k-space segments. The separation of the coherence pathway images is coupled closely with simultaneous multislice (SMS) parallel imaging methods. MR-CPU represents a novel method for adding value to an MRI examination by adding value to an MRI examination through efficient multi-contrast—and potentially quantitative—imaging.Theory
In the pulse sequence shown in Figure 1a, five spin echoes arise from separate coherence pathways. At times $$$t=4\tau$$$ and $$$t=6\tau$$$, two echoes form at the same time (i.e., $$$E_2+E_3$$$ at $$$t=4\tau$$$ and $$$E_4+E_5$$$ at $$$t=6\tau$$$). Without the use controlled aliasing methods, the contrast attributed to each of these echoes will be indistinguishable in the final reconstructed image that is representative of the sum of the individual echo amplitudes. Using the extended phase graph formalism, we determined an RF phase cycling schedule (Figure 1b) that allows CAIPIRINHA-like phase cycling of one of the superimposed coherence pathways relative to the other. Applying the phase cycling schedule over k-space segments as shown in Figure 1b provides the additional encoding capability necessary to separate the entangled coherence pathways during image reconstruction.Methods
A 2D version of the sequence in Figure 1a was programmed on a 3T system (Verio, Siemens Healthineers, Erlangen, Germany). Cartesian MR-CPU phantom experiments were performed to ensure consistent contrast between simultaneously and individually acquired coherence pathway images. Cartesian and radial MR-CPU scans were performed in a consenting brain cancer patient. A conjugate gradient SENSE reconstruction algorithm similar to SMS SENSE methods was written in Python to separate the coherence pathway images.Results
The results of the comparison between MR-CPU-derived and separately acquired $$$E_2$$$ and $$$E_3$$$ phantom images can be seen in Figure 2. The aliased $$$E_3$$$ and $$$E_4$$$ images shown in the left part of the figure show the FOV/2 shift between the two pathways, as expected with the RF phase modulation performed along the phase encoding dimension. Following the Cartesian SENSE reconstruction, the pathway images were successfully separated. The MR-CPU-derived pathway images, visually, are quite representative of the separately acquired spin echo ($$$E_2$$$) and stimulated echo ($$$E_3$$$) reference images. A regression of the pixel magnitudes between each pair of images are shown. Very strong $$$R^2$$$ values of 0.9997 and 0.9993 for the $$$E_2$$$ and $$$E_3$$$ images, respectively, demonstrate a robust agreement between the MR-CPU and reference images. Furthermore, the lines of best fit between the MR-CPU and reference images, shown in Figure 2, have slopes close to one with minuscule intercepts.The in vivo brain results are shown in Figure 3. Each of the pathway images $$$E_1$$$ through $$$E_5$$$ demonstrate unique contrast. $$$E_1$$$ is predominantly proton density weighted, $$$E_2$$$ is $$$T_2$$$-weighted, and $$$E_3$$$ through $$$E_5$$$ demonstrate mixed $$$T_1$$$- and $$$T_2$$$-weighting. No artifacts from the Cartesian SENSE reconstruction are apparent in the MR-CPU images. Given the minimal levels of acceleration employed for the radial k-space coverage (256 projections acquired out of approximately 402 required to reach the Nyquist rate), streaking artifacts arising from radial sampling in the MR-CPU images are barely visible.
Discussion
The results from this feasibility study of magnetic resonance coherence pathway unraveling demonstrate a novel mechanism for obtaining multi-contrast information from a single pulse sequence. The unique phase of each coherence pathway was modulated between k-space segments to control the aliasing of the overlapped coherence pathway signals. The controlled aliasing was exploited to separate entangled coherence pathway images with Cartesian and non-Cartesian sensitivity encoding reconstructions.Acknowledgements
No acknowledgement found.References
- Breuer et. al. Mag. Reson. Med. 55: 549-556 (2006).
- Pruessman et. al. Mag. Reson. Med. 46: 638–651 (2001).
Figures

(a) Acquisition timing diagram between (top) applied RF pulses (middle) crusher gradients $$$G_C$$$, and the crusher gradient-induced phase for the five pathways relevant to this 4-pulse experiment. (b) The RF phase cycling scheme used in this work modulates the phase of $$$E_3$$$ and $$$E_5$$$ by $$$\pi$$$ between k-space segments, but does not modulate the phase of $$$E_2$$$ and $$$E_4$$$. Here, $$$F^H$$$ represents the adjoint Fourier transform operation, and $$$\Phi^H_{E_e}$$$ adds the complex conjugate of the RF-induced phase to the $$$e^{th}$$$ pathway.
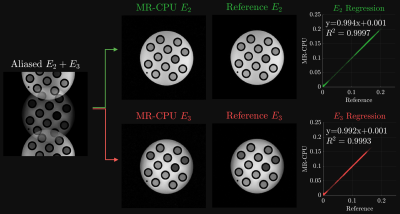
Comparison of separately acquired reference and MR-CPU-derived $$$E_2$$$ and $$$E_3$$$ pathway images. The left-most image depicts the aliased $$$E_2$$$ and $$$E_3$$$ images prior to MR-CPU separation demonstrating the inter-pathway field-of-view shift. The right side of the figure depicts the comparison between MR-CPU images and the separately acquired pathway images. The regression results in each case show a very strong agreement between the reference and MR-CPU pixel magnitude measurements. The image intensities plotted have arbitrary units.
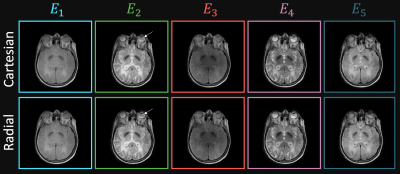
In vivo MR-CPU results from the brain of a healthy volunteer. Multi-coherence pathway images were reconstructed successfully with both Cartesian and radial k-space trajectories. Each image is windowed separately for display. The arrow indicates the presence of barely visible streaking artifacts in the radial image that are not present in the Cartesian image.