S75
Does computer-aided localization of a breast lesion impact Breast MRI-guided biopsies compared with manual localization?1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
An MRI-guided breast biopsy can be lengthy and uncomfortable for the patient, which potentially can be mitigated by computer-aided software that helps localize the lesion, compared with manual localization. We performed a retrospective trial to see if a computer-aided software system reduces procedure length, increases accuracy, and increases confidence of the clinician performing the procedure. Our study demonstrates that computer-aided software can significantly decrease length of time of the procedure but does not impact accuracy of the biopsy or confidence of the clinician performing the procedure.
Background
MRI-guided vacuum-assisted breast biopsies are performed to sample suspicious lesions not seen on conventional imaging (1)(2). For a biopsy, a woman lies on the MRI table in breast coils, with breast in compression, while multiple scans, administration of contrast, and localization of the lesion via a grid and skin marker are performed (3). The procedure can be lengthy and uncomfortable for the patient, which potentially can be mitigated by a computer-aided software to localize the lesion, decreasing patient's time of discomfort, compared to manual localization, which may be prone to human error and increase intraprocedural time (4). We performed a retrospective trial to see if computer-aided software system reduces procedure length, increases accuracy, and increases confidence of the clinician performing the procedure.Materials and Methods
MRI-guided biopsy with localization:A Sentinelle Table is connected to the 1.5 Tesla 450W GE Discovery MRI Unit which uses the DV 26 software (Figure 1). The breast is then adequately compressed to prevent it from moving but at the same time allow flow of contrast into the breast. The center of the grid is then marked within the interventional grid. With manual localization, a vitamin E marker is placed on the patient's skin within the grid (Figure 2). Alternatively with a computer-aided software, the manufacturer's special fiducial marker is placed (Figure 3). This fiducial marker is substantially larger than the common vitamin E marker.
With computer-aided software, entire coverage of the manufacturer's fiducial is important as localization will otherwise fail. Localizing the lesion manually is done with the knowledge of the breast lesion position relative to the vitamin E marker within the grid. Depth is calculated by subtracting the image number with the lesion from the image number with skin and multiplied by the slice thickness. A computer-aided software takes all these parameters into account and provides the location of the lesion relative to the fiducial and depth of the lesion. Once the lesion is localized, a needle holder is placed, which itself is a small grid to help optimally target the lesion in smaller increments. The needle is then advanced into the breast and its positioning and sampling confirmed with before and after T1 post contrast images.
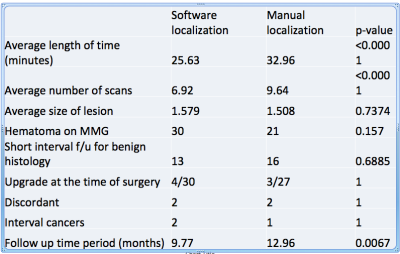
Retrospective study was performed with evaluation of electronic medical records and imaging of 100 sequential women biopsied under MRI-guidance with manual localization and 100 sequential women biopsied under MRI-guidance with computer-aided software, from December 2018 extending retrospectively to January 2016. Information regarding procedure time, number of scans, histology, post procedure hematoma, follow-up recommendations, outcome of surgery if performed, interval cancers, and follow up period was collected (Figure 4).
Results
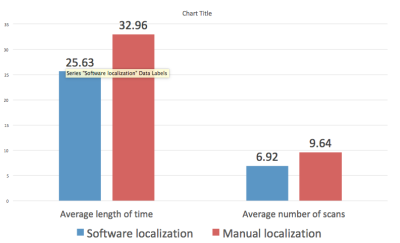
Retrospective study demonstrates a significant decrease in both average procedure time and number of scans with computer-aided software compared with manual localization (25.63 minutes vs. 32.96 minutes, p = <0.001 and 6.92 vs. 9.64, p = < 0.001, respectively) (Figure 4). There was no difference in size of breast lesions (computer aided software 1.57 cm vs. manual 1.508 cm, p = 0.7374) (Figure 5). Post procedural hematoma rate was similar between both groups (computer aided software 30% vs. manual 21%, p = 0.157). Rates of short interval follow up after benign histology a surrogate for performing clinicians confidence, was not significantly different (computer aided software 13% vs. manual 16%, p = 0.6885). There was also no significant difference in upgrade rates at the time of surgery, discordant results, and interval cancers. Follow up period was significantly longer in the manual cohort compared with the computer-aided software cohort (12.96 months vs. 9.77 months, respectively).Teaching points
- The protocol uses LAVA VIBRANT-Flex for both sagittal and axial views
- Sagittal protocol: TR of 17.2ms, TE of 4.2ms, slice thickness of 3mm; matrix size 256 x 192, field of view (FOV) 34-36, bandwidth of 200KHz, flip angle 15.
- Axial protocol: TR of 5.1ms, TE of 2.4ms, slice thickness of 2mm, matrix size of 256 x 160, FOV of 24-26, bandwidth of 41.67KHz, flip angle of 10.
- Localizing the lesion manually is done with the knowledge of the position of the vitamin E marker and the breast lesion within the grid and the depth of the lesion from the skin. With computer-aided software, the manufacturer’s fiducial is used. Entire coverage of the fiducial is important as localization will fail.
- Computer-aided software may decrease procedure time compared to manual localization.
Summary
MRI guided biopsy is a vital technique to help sample breast lesions not seen with conventional imaging. Procedural imaging parameters are well established. Tools such as computer-aided software can potentially help mitigate a lengthy procedure and patient discomfort. However, it does not impact accuracy of the biopsy or confidence of the clinician performing the procedural. Limitations of the study include small retrospective study and inherent bias.Acknowledgements
DISCLAIMER
This disclaimer informs readers that the views, thoughts, and opinions expressed in this text solely to the author, and not necessarily to the author’s employer, organization, committee or other group or individual. We do not have any affiliation with the Hologic company other than what is described within this content.
References
1.Boo-Kyung Han, Mitchell D. Schnall, Susan G. Orel, and Mark Rosen. Outcome of MRI-Guided Breast Biopsy. American Journal of Roentgenology 2008 191:6, 1798-1804
2.Chevrier M-C, David J, Khoury ME, Lalonde L, Labelle M, Trop I. Breast biopsies under magnetic resonance imaging guidance: challenges of an essential but imperfect technique. Curr Probl Diagn Radiol 2016;45(3):193-204.
4.Gombos EC, Jagadeesan J, Richman DM, Kacher DF. Magnetic Resonance Imaging-Guided Breast Interventions: Role in Biopsy Targeting and Lumpectomies. Magn Reson Imaging Clin N Am. 2015;23(4):547–561. doi:10.1016/j.mric.2015.05.004
3.Raza S, Chikarmane SA, Gombos EC, Georgian-Smith D, Frost EP. Optimizing Success and Avoiding Mishaps in the Most Difficult Image-guided Breast Biopsies. Seminars in Ultrasound, CT and MRI 2017;0(0).
Figures
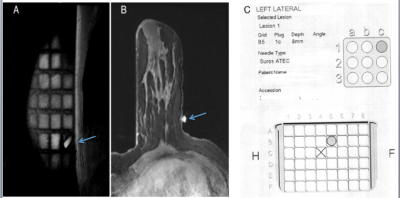
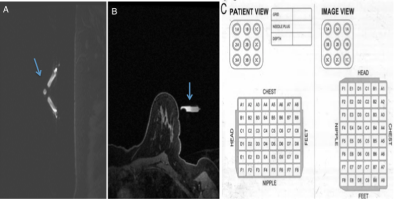
Figure 3. Pre-contrast sagittal (A) and axial (B) view demonstrating computer-aided software localization with manufacturer's fiducial marker (arrow). C. is a generated localization.