S55
Tiny Bubbles versus Spinning Protons: Can Contrast-Enhanced Transfontanelle Ultrasound (ce-TUS) Compete with MRI in Neonatal Brain Imaging?1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Aureus Medical Group, Omaha, NE, United States
Synopsis
MRI is considered to be the current gold standard for neonatal brain imaging. However, there are often challenges when transporting critically ill neonates for diagnostic imaging studies including clinical instability, safety related to patient transport, the possible need for sedation, and MR incompatibility of patient monitoring equipment. Our study retrospectively examined standard brain MRI to contrast-enhanced transfontanelle ultrasound (ce-TUS) in neonates.
Background
MRI is considered to be the current gold standard for neonatal brain imaging because of its high specificity and sensitivity1. However, there are often challenges when transporting critically ill neonates for diagnostic imaging studies including clinical instability, safety related to patient transport, the possible need for sedation, and MR incompatibility of patient monitoring equipment. Timely diagnosis is imperative for appropriate treatment, and although diagnostic accuracy is higher in MRI, cranial ultrasound is often used to provide a rapid evaluation of these acutely ill patients. Ideally, intracranial abnormalities could be accurately diagnosed bedside, with portable MRI, but that is presently not an option. Therefore, the use of contrast-enhanced ultrasound may be a viable alternative to bridge the gap2 until the neonate can be safely transported for an MRI scan. The purpose of our study was to retrospectively examine standard brain MRI to contrast-enhanced transfontanelle ultrasound (ce-TUS) for efficacy and accuracy in neonates from within our Neonatal Intensive Care Unit (NICU).Methods
Inpatients at UPMC Children’s Hospital of Pittsburgh NICU were monitored for the completion of a clinically prescribed brain MRI on one of our institution’s MRI systems: a GE 1.5T SIGNA™ HDxt, a GE 3T SIGNA™ Architect (GE Healthcare, Waukesha, WI) or a Siemens 3T MAGNETOM™ Skyra (Siemens, Erlangen, Germany). A standard protocol was acquired according to the patient’s history and diagnosis with the minimum sequence requirements for cross-comparison shown in Table 1. There were no restrictions on minimum / maximum slice thickness, field of view or acquisition matrix. Imaging was performed utilizing the corresponding system’s head array-coil. Neonates were prospectively recruited within 24 hours of MRI scan completion according to guidelines set forth by our Institutional Review Board (IRB). Signed informed consent was obtained from both parents. A GE Loqiq™ E9/E10 2.0 ultrasound system (GE Healthcare, Chicago, Illinois) was utilized and Lumason® (sulfur hexafluoride lipid-type A microspheres; Bracco S.p.A, Milan, Italy) contrast agent was administered intravenously, as an off-label application. The microbubbles are comprised of an inert gas encapsulated by a lipoprotein shell, are smaller than erythrocytes, and are exhaled via the lungs. Contrast was injected intravenously through a functioning IV. Dynamic imaging began at the time of injection (wash-in) and continued until contrast wash-out, or less than 5 minutes. The radiologist performing the ce-TUS examination was blinded to the results of the MRI at the time of acquisition. The ce-TUS images were then compared to the MRI for accuracy of diagnosis.Results
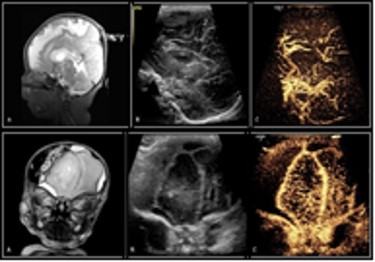
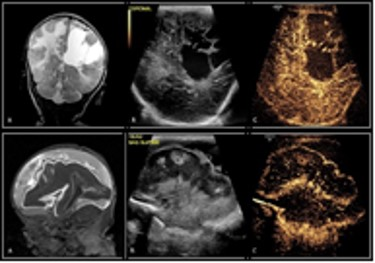
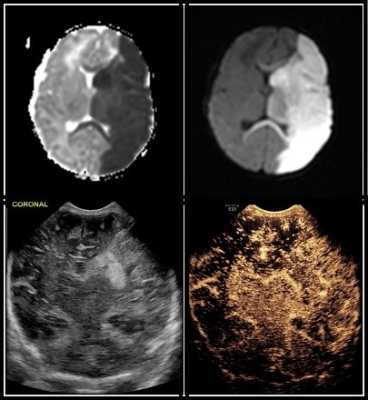
A total of 23 neonates (median PCA of 38.57 weeks) received contrast-enhanced transfontanelle ultrasound following a clinically indicated brain MRI (for rule out: 6 infection, 6 seizures, 4 HIE, 4 known anomalies, 2 follow-ups, 1 prematurity) with the median time for ce-TUS completion at 17.5 hours post MRI. Concordance of interpretation (Figures 1 and 2) occurred between ce-TUS and MRI in 14 of the 23 subjects scanned (61%) with 6 of these subjects determined to have otherwise normal results in both. Discordance occurred in 8 subjects* (35%) with findings of acute infarct, posterior subdural bleeds, ventricular hemorrhage, clinically insignificant calcifications, hemorrhagic transformation of ischemia, focal hyperperfusion and T2 hyperintensities being seen on MRI alone (Figure 3). While ce-TUS was able to make the diagnosis of left MCA infarct luxury perfusion (hyperperfusion) on 1 subject (4%) which was not perceived on the MRI images (Figure 4). (*Of note, the focal hyperperfusion and T2 hypersensitivities reported on the first brain MRI and thusly tracked as discordance between the MRI findings and ce-TUS findings, were not present on the follow-up brain MRI.)Conclusion
Our goal was to determine the possibility of imaging critically ill neonates, at bedside, with the same or similar diagnostic accuracy of MRI. We were able to show that ce-TUS is able to provide a direct view of intracranial vascularization patterns, which may provide a rapid diagnosis of cerebral ischemia, perfusion, hyper- or hypo-vascular masses, and improve visualization of extra-axial hemorrhage. While ce-TUS was able to detect brain perfusion abnormalities in the setting of injury, it still has limitations of not being able to image the cerebellum and brainstem. Further study is needed to evaluate the sensitivity and specificity of ce-TUS, and until that time, MRI will continue to be the gold standard in neonatal.Acknowledgements
Vince Kyu Lee and Christine Johnson - Pediatric Imaging Research Center; the Clinical MRI and Ultrasound Staff at UPMC Children’s Hospital of Pittsburgh - Department of Radiology.
Supported by the Society of Pediatric Radiology Research and Education Foundation Seed Grant.
References
1 Epelman M, Daneman A, et al. Neonatal Encephalopathy: A Prospective Comparison of Head US & MRI. Pedi Radiol 2010; 40:16, 40-50
2 Vos HJ, de Jong N, et al. Future applications of advanced neonatal cerebral ultrasound. Paediatrics and Child Health 2017; 28:1, 28-33
Figures