S53
Multiple Targets for Drug Infusion Using Real-Time Convection-Enhanced Delivery in NHP Model for Therapies of Neurodegenerative Disorders
Anna M Borkon1, Michael J DiBalsi2, Megan Keiser3, and Timothy H Lucas4
1Research, University of Pennsylvania, Philadelphia, PA, United States, 2ClearPoint, ClearPoint, MRI Interventions, INC., Irvine, CA, United States, 3The Center for Cellular and Molecular Therapy, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
1Research, University of Pennsylvania, Philadelphia, PA, United States, 2ClearPoint, ClearPoint, MRI Interventions, INC., Irvine, CA, United States, 3The Center for Cellular and Molecular Therapy, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
The current technological advances of the intracranial
Convection-Enhanced Delivery (CED) open a novel possibility for infusing
therapeutics directly into multiple targets located in the same cerebellar
hemisphere with high precision and safety. The presented experiment is based on
the AAV1 infusion into the Deep Cerebellar Nuclei (DCN), namely the dentate,
interposed and fastigial in NHPs using the real-time CED approach. In retrospect,
the conclusion of this experiment is that multiple targets located close to
each other in the brain can be successfully infused during the same MRI session
with no or minimal reflux visualized on the post-infusion images.
PURPOSE
In the efforts to search for effective treatment of neurodegenerative disorders, this experiment uses the NHP model to test if multiple targets located close to each other in the brain can be successfully infused during the same MRI session. AAV1 was successfully infused into the Deep Cerebellar Nuclei (DCN), namely the dentate, interposed and fastigial (Fig.1.) using the real-time Convection-Enhanced Delivery (CED) approach.MATERIALS AND METHODS
AnimalsThe experiment was performed on 25 Rhesus Monkeys (Macaca Mulatta), 3.0–8.0 kilograms, 3+ years old at the time of surgery and approved by the National Institutes of Health (NIH) and the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
Preoperative Procedure
The monkeys underwent sedation with Telazol (3-5 mg/kg) and general anesthesia (Isoflurane) with endotracheal intubation. Each monkey’s head was shaved and sterile lubricant was placed in their eyes. Routine physiological monitoring was established.
Test Article and Vehicle
Test article adeno-associated virus serotype1 (AAV1) was delivered to the DCN by a programmed infusion pump (Harvard Apparatus) at a rate between 1-10 μl/min. The dose of 2.6×10¹²vg was selected based on previously published efficacy data on mice and NHP¹.
Surgical Procedure in Real-Time
The surgery was performed under direct MRI guidance using an FDA-approved stereotactic system. First, the primate’s head was placed supine into Clear Point’s 4-point cranial fixation frame (SmartFrame) and attached to the right and left skull hemispheres with 4 screws percutaneously. The monkey’s body was covered with a warm blanket, secured with a strap and moved into the bore of the 3T Siemens Magnetom for a 3-plane localizer scan to assess the head positioning. The scanning table was then moved to the back of the magnet and antibiotics Cefazolin and Dexamethasone (0.25-1 mg/kg IM up to BID) were administered. The head was prepped with Chlorhexidine and the sterile field was established. A contrast-filled grid was placed on the skull to identify the entry point for the planned trajectories. Next, a high-resolution T1 weighted 3D MPRAGE scan of the whole head was acquired for the surgical planning. The obtained images were transferred to ClearPoint software for an anterior commissure – posterior commissure (AC-PC) determination and trajectory planning. The images were acquired with MR-visible fiducial markers to pinpoint the target location with greater accuracy. Next, 3D T2 weighted images were acquired and transferred to the software to provide better visualization of the target. Following trajectory planning, the ClearPoint base and tower were mounted directly to the scull over the entry point provided via the software. A hand controller was also mounted to the tower, allowing the surgeon to adjust the trajectory to the desired target location in 4-planes using the ‘pitch and roll’ and X-Y transitional axes. The primate was moved to the isocenter of the bore and Fast Gradient Echo scans were obtained to detect the position and orientation of the cannula. The acquired images were used by the software to compare the current trajectory to the target trajectory to calculate an expected error for cannula tip placement and estimate the new parameters for the cannula alignment adjustment using the pitch and roll (Fig. 2). Once the final adjustment was completed, a 3.4 mm hole was made in the scalp (<1 cm) using an MR-safe twist drill. Next, the cannula was measured out to target depth and advanced to the first target (Fig. 3). The infusion of AAV1 to the first target started, and 3D MPRAGE was acquired. The images were transferred to the software for visual reassessment of tip accuracy to the target. Once the infusion of the first target was finalized, a post-MPRAGE scan was applied to confirm the anatomical dispersion of the delivered test article. The images were transferred to the software and the drug infusion placement was visually confirmed (Fig. 4). The same process was repeated for the second and third target. After the third infusion was completed and the accuracy of the drug delivery accepted, the infusion catheter, cranial fixation base and tower were removed. Finally, a small piece of bone wax was applied in the trephine and sutures were used to close the head incision.
RESULTS
The experiment proved that the drug delivery was highly accurate and allowed the infusion of AAV1 into multiple brain targets located close to each other in the cerebellum during the same MRI session with no or minimal reflux visualized on the post-infusion images(Fig.5). In 2 cases some reflux was observed but in subsequent testing it was eliminated. Also, there were no occurrences of MRI-visible hemorrhages during cannula placement and no adverse effects after the procedure.CONCLUSIONS AND FUTURE DIRECTIONS
The Convection-Enhanced Delivery (CED) of AAV1 in real-time using the FDA-approved stereotactic system for targeted gene-based treatments in Rhesus Monkeys provides high precision and safety of drug delivery. Also, it demonstrates the novel ability to deliver controlled volumes of this drug directly to any structure in the NHP brain with accuracy on the order of <1 mm. Additionally, the capacity of CED to monitor the infusion in real-time opens opportunities for using the NHP brain to develop neurodegenerative therapies in humans.Acknowledgements
This work was supported by NIH grant.References
References 1.Keiser, M. Pre-Clinical Pharmacology/ Toxicology AAV1 miS1 Dosing Study in Non-Human Primates. Philadelphia: Protocol; 2015Figures
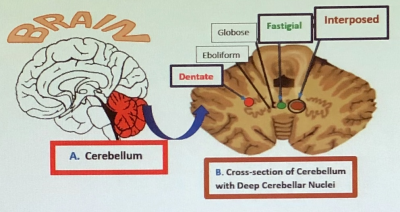
Figure 1. Cerebellum: (A) Location. (B) Deep Cerebellar Nuclei (DCN). It controls movement coordination, balance, equilibrium and
muscle tone.
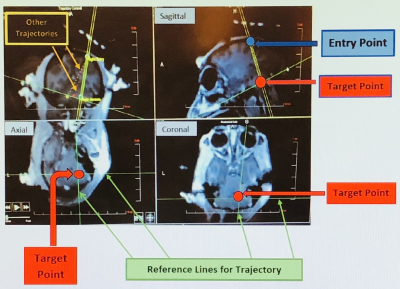
Figure
2. MRI images of the
monkey’s head in sagittal, axial and coronal orientations used for cannula tip placement and trajectory alignment.
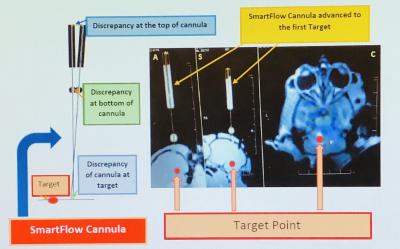
Figure
3. The SmartFlow cannula
adjusted to the desired target trajectory. The monkey’s head in (A) axial, (S)
sagittal and (C) coronal plans.
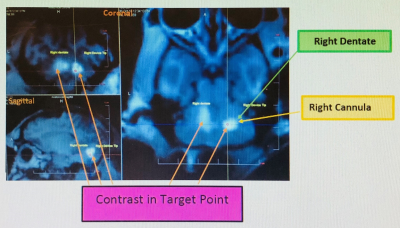
Figure
4. Post-infusion 3D MPRAGE T1 weighted
image in sagittal and coronal orientations for drug infusion placement visualization.
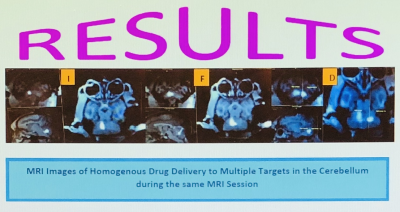
Figure
5. Homogenous drug
infusion delivery to the DCN: (I) interposed, (F)
fastigial and (D) dentate on sagittal, axial and
coronal images.