S50
Plugging the gap: A reassessment of gadolinium administration policy in patients of unknown pregnancy status1Radiological Sciences, Singapore General Hospital, Singapore, Singapore
Synopsis
The administration of gadolinium-based contrast agents in magnetic resonance imaging (MRI) is traditionally avoided during pregnancy, due to safety concerns over gadolinium exposure to the fetus. In our institution, female patients of reproductive age are required to undergo a urine pregnancy test (UPT), should contrast-enhanced MRI be required. However, this policy is not sufficiently comprehensive in safeguarding patients in the early stages of pregnancy as a UPT can be inaccurate between days 14 to 28 of the menstrual cycle. This presentation will reassess and propose changes to the gadolinium administration policy in patients of unknown pregnancy status in our institution.
Background
The administration of gadolinium-based contrast agents (GBCAs) in magnetic resonance imaging (MRI) is traditionally avoided in pregnancy across many institutions globally. This is primarily due to potential deleterious effects of GBCAs on the fetus1. In our institution, female patients of reproductive age between 9 to 60 years old, whose last menstrual period exceeds 28 days, are required to undergo a urine pregnancy test (UPT), should contrast enhanced MRI be required. However, this policy is not sufficiently comprehensive in safeguarding patients in the early stages of pregnancy (less than 28 days). Additionally, the accuracy of UPT results is debatable between days 14 to 28 of the menstrual cycle2. There is therefore a need to reassess and propose changes to the gadolinium administration policy in patients of unknown pregnancy status in our institution.Teaching Point
This presentation aims to1. Highlight the potential effects of GBCA on fetus in pregnancy.
2. Review and propose changes to the current gadolinium administration policy in patients of unknown pregnancy status in SGH.
Summary
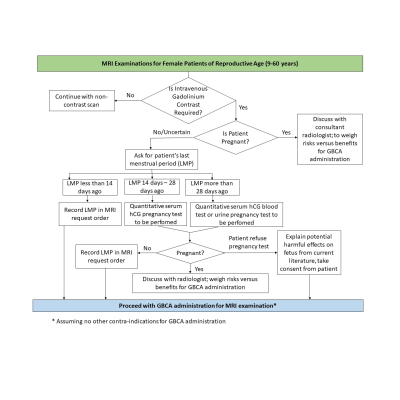
A review of the literature from 2013 to 2019 regarding the accuracy of pregnancy tests, recommendations, and effects of gadolinium use in pregnancy was conducted using the PubMed library database, as well as various reputable journals such as Radiology and Journal American Medical Association. The sampling period selected was due to the sudden surge in number of studies investigating the effects of gadolinium, after the discovery of gadolinium deposition in the brain from use of GBCAs in recent years. Documents regarding the institution’s department policy guidelines pertaining to pregnancy were also reviewed. In addition, the department’s workflow for high dose ionizing radiation examinations of the abdominal or pelvic region for female patients of reproductive age was also used as supplementary material, in proposing changes to the current gadolinium administration policy for potentially pregnant patients.Studies performed on both humans and animals have shown that GBCAs pass through the placental barrier, with trace amounts of gadolinium remaining in the fetal circulation, amniotic fluid and fetal tissues3. While most studies showed no adverse effects to the fetus associated with gadolinium use in pregnancy, the long-term effects have yet to be investigated and are very much unknown. A recent study involving more than 1.4 million pregnancies in Canada, reported an increased relative risk for stillbirth and neonatal death, and increased risk of rheumatological, inflammatory, or infiltrative skin conditions in new-borns who were exposed to gadolinium during pregnancy1. Meanwhile, another study investigating the incidence of gadolinium exposure during pregnancy in the United States, identified 6879 exposures to GBCAs in a sample size of more than 4.6million pregnancies, which is about one in 860 pregnancies. In this study, the majority (70.2%) of exposures to GBCAs occurred in the first trimester, suggesting high likelihood of inadvertent exposure to GBCAs before pregnancy is recognized4. The literature also showed quantitative serum pregnancy test to be of higher sensitivity as compared to UPT, especially in the early stages of pregnancy2. As such, proposed changes to current policy, for example the checking of last menstrual period prior to day of scan and ordering for quantitative serum pregnancy test, will be discussed (Figure 1). The proposed changes may be used as a reference for other institutions around the world with similar pregnancy screening policies.
Acknowledgements
None.References
1. Ray, Joel G., et al. "Association between MRI exposure during pregnancy and fetal and childhood outcomes." Jama 316.9 (2016): 952-961.
2. Tirada, Nikki, et al. "Imaging pregnant and lactating patients." Radiographics 35.6 (2015): 1751-1765.
3. Prola-Netto, Joao, et al. "Gadolinium chelate safety in pregnancy: barely detectable gadolinium levels in the juvenile nonhuman primate after in utero exposure." Radiology 286.1 (2017): 122-128.
4. Bird, Steven T., et al. "First-trimester exposure to gadolinium-based contrast agents: a utilization study of 4.6 million US pregnancies." Radiology 293.1 (2019): 193-200.