S44
Multiband and Multi-echo EPI – the good, the bad and the future of fMRI at ultra-high field1MBCIU, The University of Melbourne, Parkville, Australia, 2Royal Melbourne Hospital, Melbourne, Australia
Synopsis
Functional MRI is a dominant tool in both neuroscience research and neurosurgical planning. Multiband EPI can not be ignored as a staple acquisition tool for fMRI. It has the ability to assist in improving temporal resolution, spatial resolution and signal to noise. When using this highly effective and efficient technique there are considerations in order to gain the most for the sequence without producing detrimental imaging artefacts. Once the technique is understood there is a future application of Multiecho Multiband EPI which can potentially be used with great success to increase sensitivity and specificity of BOLD signal and fMRI.
Background
Functional Magnetic Resonance Imaging (fMRI) uses Echo Planar Imaging (EPI) to measure Blood Oxygen Level Dependent (BOLD) response in the brain to neural activity. The research applications are wide and varied, particularly for cognitive neuroscientists, however fMRI is also increasingly used in clinical practice for improved neurosurgical planning.The acquisition of this data has been somewhat and modestly optimised over the years as field strengths increased, and RF and gradient technology improved. More recently Multiband (MB) MRI, also known as Simultaneous Multi Slice (SMS), has allowed for multiple slice locations to be excited simultaneously. The use of this technique in conjunction with EPI and multi-channel receive coil reconstruction techniques has led to a dramatic improvement in fMRI, including increased spatial (< 1mm isotropic) and temporal resolution (TR<1s), while maintaining whole brain coverage at 7T [1, 2]. It has also allowed for the introduction of Multi-Echo fMRI which can be a successful way to increase the BOLD signal and contrast in small sub-cortical brain structures.
Teaching Point
1. The addition of SMS to EPI means whole brain coverage can be achieved with sub millimetre slices and sub second temporal resolution for fMRI at 7T.Whilst the brain is exposed to a task there are changes in the blood flow in capillaries of the cortex . BOLD imaging uses the susceptibility difference oxyhaemoglobin and deoxyhaemoglobin to quantify brain activation. Timing of the acquisition is vital in imaging these changes in blood flow and with this requirement comes the consideration of TR (which dictates temporal resolution), voxel size (spatial resolution) and TE (contrast) [3, 4]. Correct TE selection is vital as the BOLD effect relies on T2* weighting differences between deoxyhaemoglobin and oxyhaemoglobin. This T2* effect increases with the increase in magnetic field leading to significant increases in BOLD contrast at ultra-high fields (7T or higher) [5] (Fig 1). This together with the increase in MRI signal to noise ratio (SNR) at 7T means there is the possibility to explore improved spatial and temporal resolution to sub millimetre, sub second (Fig 2). However, with thinner slices, associated with higher resolution images, whole brain coverage becomes a major issue. The introduction of multiband has made more coverage possible.
The Good- Multiband imaging uses composite RF pulses to simultaneously excite multiple slice locations through the use of slice selective gradients [6]. The MB factor determines how many slices are acquired within one TR. A high MB factor can increase the coverage or inversely allow a reduced TR to improve temporal resolution [7]. One advantage is that because thinner slices can be achieved there is less through slice dephasing which appears as signal dropout in the frontal and hippocampus areas using traditional fMRI [8] (Fig 2). These advantages of whole brain coverage with higher spatial and temporal resolution through the acquisition with multiband can be seen in a comparison of whole brain imaging at 3T and 7T (Fig 3).
The Bad – Using multiband doesn’t come without challenges. Both artefacts and significantly reduced SNR can be produced if the MB factor is too high [9]. This occurs when the simultaneously excited slices become too close together and the receive coil array fails to adequately separate the signals. When stacked slices are viewed from the perpendicular plane one sees significant slice banding (Fig 5a).
2. Adding multiple echoes to the MB-EPI sequence further increases fMRI signal and contrast.
The Future - As we move forward with fMRI and the improvements of BOLD imaging with MB we have seen the introduction of Multi Echo fMRI. Recently ME fMRI has moved from a technique being itself researched to both a research and potentially clinical technique. It involves the acquisition of multiple echoes within one TR on simultaneously excited slices [7]. This technique has potential to dramatically improve fMRI for two reasons: 1. The sum of the echoes increases SNR. 2. Optimal BOLD contrast is when the TE = T2*, therefore multiple TE’s will increase BOLD contrast [10]. Since different gray matter structures have a different T2* (particularly sub cortical) there are more chances of capturing the highest BOLD contrast from as many tissues as possible (Fig 4). The combination of the signals across the multiple TEs can then produce a higher sensitivity than a single TE fMRI [10]. This is supported in cases where there is signal dropout from susceptibility artefact in one TE but not in another. (Fig 5b.) It can also mean activation in subcortical layers can be separated by the different activation times for increased specificity [11].
Summary
The addition of MB to EPI means that higher temporal and spatially resolved fMRI can be realised without compromise of spatial coverage. In addition, combining this with a multi-echo readout can further increase the BOLD contrast, particularly for sub-cortical grey matter structures, increasing the sensitivity and specificity. This can be of great assistance to fMRI and the advancement to applied neuroscience and clinical neuroscience research. In principle, the MB technique can work for any slice selective sequences and as such the technology could be used to decrease slice thicknesses without compromise of coverage for a whole range of applications.Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of the Australian National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Melbourne Brain Centre Imaging Unit of the University of Melbourne. The work was also supported by a research collaboration agreement with Siemens Healthineers. Acknowledgment also to Centre for Magnetic Resonance Research, Minnesota for the development and supply of the Multiband EPI sequence required for this project.References
1. Benedikt A, Poser B, Setsompop K. Pulse sequences and parallel imaging for high spatiotemporal resolution MRI at ultra-high field. NeuroImage, Volume 168, March 2018, Pages 101-118
2. Feinberg DA, Yacoub E. The rapid development of high speed, resolution and precision in fMRI. Neuroimage Volume 62, 2012. Pages 720–725.
3. Demetriou L, et al. A comprehensive evaluation of increasing temporal resolution with multiband-accelerated protocols and effects on statistical outcome measures in fMRI. NeuroImage., Volume 176, 2018, Pages 404–416
4. Menon RS, et al. BOLD based functional MRI at 4 T includes a capillary bed contribution: echo planar imaging correlates with previous optical imaging using intrinsic signals. Magn. Reson. Med. Volume 33, 1995. Pages 453–459.
5. Moeller S, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn. Reson. Med. Volume 63, 2010. Pages 1144–1153.
6. Barth M, Breuer F, Koopmans PJ, Norris DG, Poser B. Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med. Volume 75, 2015. 63–81.
7. Boyacioglu R, Schulz J, Koopmans PJ, Barth M, Norris DG. Improved sensitivity and specificity for resting state and task fMRI with multiband multi-echo EPI compared to multi-echo EPI at 7T. Neuroimage, Volume 119, 2015, Pages 352–361.
8. Risk B, Kociuba MC, Rowe D. Impacts of simultaneous multislice acquisition on sensitivity and specificity in fMRI. NeuroImage, Volume 172, 2018, Pages 538–553.
9. Chen L , et al. Evaluation of highly accelerated simultaneous multi-slice EPI for fMRI. NeuroImage, Volume 104, 2015, Pages 452–459
10. Puckett AM, Bollman S, Benedikt A, Palmer J, Barth M, Cunnington R. Using multi-echo simultaneous multi-slice (SMS) EPI to improve functional MRI of the subcortical nuclei of the basal ganglia at ultra-high field (7T). Neuroimage, Volume 172, 2018, Pages 886-895.
11. Poser BA, Norris DG. Investigating the benefits of multi-echo EPI for fMRI at 7T. Neuroimage, Volume 45, 2009. Pages 1162–1172.
Figures
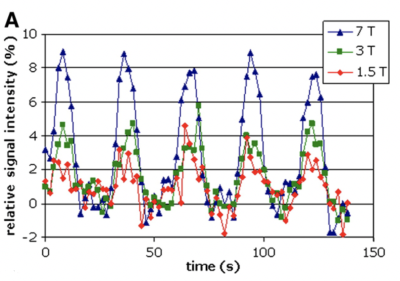
Figure 1: Signal intensity v Time at 1.5T, 3T and 7T.
Wietske van der Zwaag, et al. NeuroImage, Volume 47, Issue 4, 2009.

Figure 2: A comparison of standard 3T and 7T fMRI data.
3T Siemens, Prisma – 3mm isotropic, TR = 3000ms, TE = 30ms, FA = 85 (Royal Melbourne Hospital)
7T Siemens Magnetom - 1.6mm isotropic, TR = 800ms, TE = 22ms, FA = 45 (University of Melbourne)
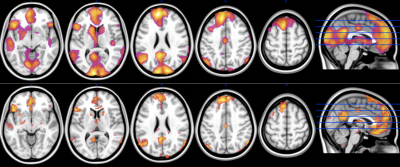
Figure 3: 3T and 7T images showing the increased specificity of 7T imaging within the grey matter.
Top – 3T GE Signa - 3.5mm isotropic, TR = 2000ms, TE = 35ms, FA = 90, N = 96 participants.
Bottom - 7T Siemens Magnetom - 1.6mm isotropic, TR = 800ms, TE = 22ms, FA = 45, N=28 participants. (Images Courtesy of A/Prof Ben Harrison – The University of Melbourne)
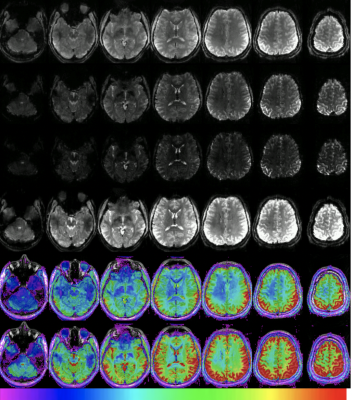
Figure 4: Multiband Multi-Echo imaging of a 27 year old male volunteer.
Rows 1 to 3 show example time frames of the 1st, 2nd, and 3rd echoes respectively. Row 4 shows an example frame of the sum of all echoes. Row 5 and 6 show the SNR of the 1st echo and sum of all echoes respectively. The colour bar indicates the SNR range from 20 to 200. Spatial resolution was 2x2x2 mm, TR/TEs were 1170/9.5,26,42 ms with a GRAPPA factor of 3, MB factor of 4.
Row 5 - Single Echo SNR of Basal ganglia = 122 +/- 22
Row 6 - Combined 3 Echo SNR of Basal ganglia = 220 +/-66
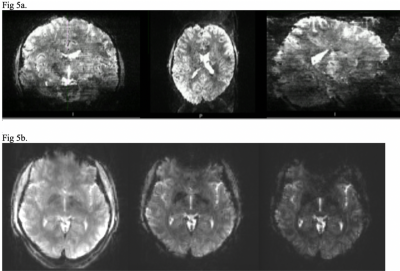
Figure 5. Artefacts
Fig 5a. Multiband banding artefact displayed as stripes throughout the image. 1x1x1.2mm voxel, TR 2500ms, TE 25ms, FA = 34, Grappa = 3, MB factor = 4
Fig 5b. Change in susceptibility artefact with a change in TE can be seen in the anterior portion of the image. TE = 10, 26, 42.