S36
Intra-individual open-label, single center study to compare unenhanced MRI with Dotarem enhanced MRI in pediatric and neonatal patient population (<18years)1Phoenix Children's Hospital, Phoenix, AZ, United States
Synopsis
The primary objective to this study was to demonstrate superiority of lesion visualization of Dotarem enhanced MRI over unenhanced MRI along with the evaluation of safety and efficacy of Dotarem enhanced MRI in pediatric and neonate patients. Safety variables as well as the assessment of adverse events (AEs) for Dotarem enhanced MRI were collected.
Background
Dotarem was introduced to the US market in 2013, however, since 1989 Dotarem has been in use in Europe and 70 other countries. The majority of the safety and efficacy data comes from Europe and the other countries. The goal of this study is to evaluate the efficacy and safety of Dotarem enhanced MRI in pediatric and neonatal population who are referred for contrast enhanced MRI. The primary objective to this study was to demonstrate superiority of lesion visualization of Dotarem enhanced MRI over unenhanced MRI. Safety variables as well as the assessment of adverse events (AEs) for Dotarem enhanced MRI were also collected.Methods and Materials
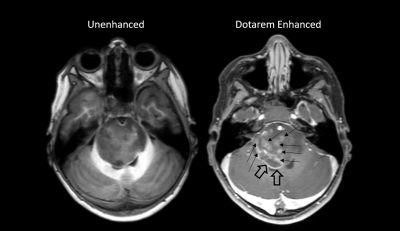
The study was designed as single center, open label comparison of 250 unenhanced MRI with Dotarem enhanced brain MRI in pediatric patient population (<18years) performed intraindividually by 3 independent blinded radiologists. All patients received standard body weight based intravenous injection of Dotarem at dose of 0.1 mmol/kg. Dotarem enhanced and unenhanced MR imaging was performed using 1.5 and 3 Tesla (T) field strength.Results
For all three readers, there was a statistically significant benefit of Dotarem contrast enhanced MRI for the detection of brain lesion border delineation (p= 0.0002, <0.0001, <0.0001) and degree of lesion contrast enhancement (p= 0.0001, 0.0003, 0.0003) compared to unenhanced brain MRI. There was a trend without statistical significance for better brain lesion morphology determination with Dotarem contrast enhanced compared to unenhanced MRI.Patients who received intravenous Dotarem contrast ranged in age from 0.1 and 24 years old (median 7 years). 36 patients were less than 1 year old (14.4%), 81 patients were 1-6 years old (32.4%), 65 patients were 6-12 years old (26%), 67 patients were 12-18 years old (26.8%), and 1 patient was greater than 18 years old (0.4%). No adverse events were reported in all 250 patients.
Conclusion
Intravenous Dotarem contrast enhanced brain MRI was superior to unenhanced brain MRI in the detection of lesion boarder delineation and contrast enhancement in a large population of pediatric patients. The intravenous administration of Dotarem was safe and without reported adverse events in the population of 250 pediatric patients including 36 patients less than 1 year of age.Acknowledgements
No acknowledgement found.References
Chachuat, A., Molinier, P., Bonnemain, B., Chambon, C., & Gayet, J. L. (1992). Pharmacokinetics and tolerance of Gd-DOTA (DOTAREM) in healthy volunteers and in patients with chronic renal failure. European Radiology, 2(4), 326-329.
Haneder, S., Attenberger, U., Schönberg, S., Loewe, C., Arnaiz, G., Michaely, H. J., ... & Santander, E. S. (2010). Intra-individual efficacy evaluation of Dotarem®-enhanced MRA compared to Gadovist®-enhanced MRA in the diagnosis of clinically significant abdominal or lower limb arterial diseases. Eur Radiol.
Miller, J. H., Hu, H. H., Pokorney, A., Cornejo, P., & Towbin, R. (2015). MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics, 136(6), e1637-e1640.
Robison, R. K., Pokorney, A., & Miller, J. H. (2019). Evaluation of the effect of switching from a linear to a macrocyclic contrast agent on the T1-weighted brain signal intensity of a child during the course of 43 contrast-enhanced MRI examinations. Journal of magnetic resonance imaging: JMRI, 49(2), 608.