S116
An exploration of MRI patient safety decision making processes using ‘social media’ as a data resource1MRI, The Florey Institute of Neuroscience and Mental Health, Heidelberg, Australia, 2Imaging, The Florey Institute of Neuroscience and Mental Health, Heidelberg, Australia, 3Imaging, The Florey Department of Neuroscience and Mental Health, University of Melbourne, Melbourne, Victoria, Australia, Melbourne, Australia
Synopsis
This study reviewed the engagement of MR Radiographers & Technologists with a social media platform seeking professional guidance with MR safety decision making processes regarding implants or devices. From June 2016 to August 2018 100 discussion items were reviewed and categorized. Our study identified that the ‘consensus to scan or not to scan’, was not completely dependent on the presence of detailed information about the implant or device.
Background
MR Radiographers & Technologists are an integral part of a team responsible for making MR safety decisions for persons undergoing MRI scans. From an MR safety perspective, an important questions ‘is whether to proceed with a scan when a patient presents with an implant in situ. The decision to not proceed can impact on the patient’s clinical care, while the decision to proceed could potentially compromise the patient’s safety within the MR environment. It is globally acknowledged that in order to proceed safely with a scan people should be screened and any implants or devices identified should be assessed to confirm their compatibility for the MR environment 1,2. A number of reference sites exist that provide information about the MR testing information and the MR safety status of an implant or device e.g. that a device or implant is MR Safe, MR Unsafe, MR Conditional and the specific MR safety conditions that apply 3-4.The pressing issue is that not all devices and implants have been MR safety tested. Furthermore, workplaces are increasingly challenging, pressure to perform MR safety checks and confirm MR safety status of implants in reduced time is common. Given these challenges, in recent years it has been noted that some MR Radiographers & Technologists are using social media to gauge opinions from others within the professional community to help them make ‘informed’ MR safety decisions. The aim of this study was to evaluate how MRI professionals are currently interacting with social media platforms to assist in MR safety decisions
Methods
In this study we evaluated information presented on the Facebook MRI Safety group social media platform. The Facebook MRI Safety group, an established social media platform with over 19 thousand members at the time of this study, is a closed group of invited contributors that are MR professionals or professionally minded individuals with an interest in MR safety. Three radiographers reviewed the implant discussion thread posted from June 2016 to August 2018 where 100 discussion items were reviewed and categorized.Results
Review of 100 discussion items - with multiple posts under each discussion item, were reviewed and categorized into three distinct areas (figure 1);Category 1: Information about standard operating procedures (SOPs) 14% of the total implant discussion thread
Category 2: General information sharing 18% of the total implant discussion thread
Category 3: Specific implant information requests 68% of the total implant discussion thread
- Sub-Category 3.1: 32 of the 68 items discussed questions about ‘to scan or not to scan?’ with ‘Make model manufacturer information of the implant details provided’
- Sub-Category 3.2: 36 of the 68 items discussed questions about ‘to scan or not to scan?’ with ‘No or non-specific information provided’.
Further review of the 68 discussion items within the 3) Specific implant information requests thread subcategories 3.1 and 3.2 yielded opinions given whether there was:
a) Clear consensus to scan
b) Clear consensus not to scan or
c) No consensus reached (due to mixed opinion)
Sub-Category 3.1: ‘Make / Model manufacturer provided’
a) Clear consensus to scan = 21 items
b) Clear consensus not to scan = 4 items
c) No consensus reached (due to mixed opinion) = 7 items
Sub-Category 3.2: No / Non-specific information provided’
a) Clear consensus to scan = 10 items
b) Clear consensus not to scan = 10 items
c) No consensus reached (due to mixed opinion) = 16 items
Discussion & Conclusion
This study reviewed the engagement of MR Radiographers & Technologists with social media to seek professional guidance to assist decision making processes regarding MR safety. On review of the of 100 discussion items posted under the ‘implant discussion thread’ there were three key areas which users frequently requested information. This included information about SOPs for Implants - where discussions were focused on specific departmental protocols when scanning specific implants such as pacemakers or neuro-stimulators. The second main area focused on ‘General Information sharing’ - where discussions focused on non-specific information about items such as new metallic inserted jewellery, or physiological sensing monitors worn by patients. These two categories totalled 32% of the dialogue and highlight the need for a MR professional community forum to exchange professional ideas, opinions and experiences.Finally, the majority (68%) of the discussion under the ‘implant discussion thread’ focused on ‘requests for professional opinions about whether to scan or not scan a particular device / implant’ (figure 2). These included a range of active and passive implanted devices. Our study identified that the ‘consensus to scan or not to scan’, was not completely dependent on the presence of detailed information about the implant or device. However, of particular interest was that opinions were provided in a number of cases ‘as to scan or not scan’ without any specific information being provided about the implant or device. This work highlights the increasing use of social media for information sourcing, however, we need to be mindful as MR professional that any information obtained and subsequent MR Safety decisions made - may have impact on both patient safety and the clinical management of the patient.
Acknowledgements
No acknowledgement found.References
1. Kanal E et al. ACR guidance document for safe MR practices (2013): Journal of Magnetic Resonance Imaging 37:501–530 2.
2. The Royal Australian and New Zealand College of Radiologists (RANZCR). RANZCR MRI safety guidelines. Sydney: RANZCR; 2017.
3. MRIsafety.com
4. fda.gov: Magnetic Resonance Imaging (MRI Safety)
Figures
Review of 100 discussion items - categorised into three distinct areas:
Category 1: Information about standard operating procedures (SOPs) 14% of the total implant discussion thread
Category 2: General information sharing 18% of the total implant discussion thread
Category 3: Specific
implant information requests 68% of the total implant discussion thread
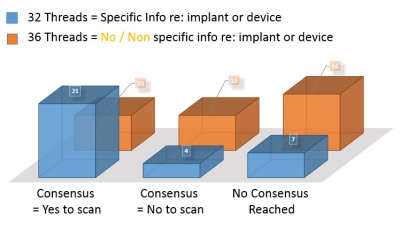
Sub-Category 3.1: ‘Make / Model manufacturer provided
a) Clear consensus to scan = 21 items
b) Clear consensus not to scan was evident = 4 items
c) No consensus reached (due to mixed opinion) = 7 items
Sub-Category 3.2: No / Non-specific information provided’
a) Clear consensus to scan = 10 items
b) Clear consensus not to scan was evident = 10 items
c) No consensus reached (due to mixed opinion) = 16 items