Implementation of Safety Checklist and Protocols for scanning patients with whole body conditional DBS implants
Nancy Talbot1 and Tanya Wah Kan1
1Joint Department of Medical Imaging, Princess Margaret Cancer Center, University Health Network, Toronto, ON, Canada
1Joint Department of Medical Imaging, Princess Margaret Cancer Center, University Health Network, Toronto, ON, Canada
Synopsis
Implementing protocols for patients with deep brain neurostimulators (DBS) that have whole body conditions can be challenging. To obtain sequences that meet the B1+rms RF deposition requirements may require modifications image quality and coverage. T1 weighted imaging can be achieved utilizing gradient echo pulse sequences, however these do not suffice for T2 weighted imaging. For T2 weighted turbo spin echo sequences multiple paramter changes are made including echo train length, slice thickness and gap, matrix, averages, flip angle and gradient operation modes. The implementation of a checklists assists the technologists in ensure conditions are met, and patient safety is ensured.
Background
MRI scanning of patients with deep brain neurostimulators (DBS) has been practice for many years. This was typically performed using and transmit receive head coil to avoid radiofrequenc (RF) deposition to the DBS pack and looped wires thereby limiting heating of these wires. Neurostimulator manufacturers have recently changed conditions for a group of DBS implants allowing for imaging of areas of the body outside of a transmit and receive head coil. The vendors have published the “whole body” criteria for this group of DBS implants. These conditions are very limiting, and therefore challenging for techologists and radiologists to achieve diagnostically appropriate images.Teaching Points
There are several procedure steps to ensure that the patient is scanned safety. This includes a detailed review of the DBS componets, documentation from the most responsible physician that the DBS is intact and functioning appropriately by using egibility sheets, and ensuring that the DBS is programmed to the appropriate mode prior to the patient entering the magnet room. Implementation of protocols that meet the criteria for the whole body conditional DBS required teaching technologists newer methods of monitoring RF. This included upgraded MRI safety education sessions to discuss B1+rms, and hands on education while scanning. B1+rms is a measurement of RF deposition for a prescribed pulse sequence, measured in uT. Unlike specific absorption rate (SAR), it is not variable by scanner vendor or patient. The values can be assessed by viewing the predicted value following the pre-scan preparation. If the value is too high, the scan is cancelled prior to starting, the parameters adjusted, and the prediction re-assessed. The conditions of the DBS whole body scanning requires the 1.5T protocols to be modified such that each sequence is below 2.0 uT or 1.2u. One DBS manufacturer stating requirements of allowing a maximum total of 30 minutes of RF deposition. To ensure that the patient is scanned safety and all criterial are met, a custom checklist, designed to be utlized throughout the exam was developed. This form allows for documentation of the steps required, as well as documentation of B1+rms and total time of RF deposition to meet manufacturers conditions.In regards to protocol development, the most common MR request has been for spine imaging. Our institutional protocols, as well as the vendor protocols did not meet the B1+rms limits as stated by the DBS manufacturer. For T1 imaging of the spine, gradient echo pulse sequences were utilized that allow for low B1+rms levels of 1.3uT. For T2 imaging, turbo spin echo sequences were modified by decreasing the echo train length from 16 to 8, moving to a low SAR mode and whisper (slow) gradient mode to increase the echo spacing. The flip angle was decreased from 150 degrees to 130 degrees. The matrix was modified from 512 read by 50% to 384 read by 75% , changing the pixel dimensions form 0.6 x 0.6 mm to 1.0 x1.0 mm. The slice thickness was increased to 4mm from 3mm with a 25% gap instead of 10%, and the averages were decreased from 2 to 1. The number of slices was decreased from 15 to 9, and the anterior saturation pulse was eliminated. The new scan time was 2:28 minutes, with a resultant B1+rms of 1.8uT. Similar parameters were utilized to achieve the axials, with addition of 1 slice and a change to 1.9uT B1+rms.
To meet the 1.2uT, the parameters for the T2 turbo spin echo sequence were modified further. This protocol also considered the needs of imaging the cervical spine which requires more detail. Once again the echo train length was decreased 18 to 8, and the RF mode of normal was changed to low SAR, along with modifications to the gradient mode from normal to whisper. The flip angle was decreased from 150 degrees to 130 degrees, and the matrix was kept at 384 read by 70% to maintain a pixel size of 0.6 x 0.6mm. The slice thickness was left at 3mm, again to maintain resolution, however the gap was increased from 10% to 25%. The averages were again decreased from 2 to 1. With these modifications the resultant number of slices that could be achieved was 3, wereas in the routine protocol we prescribed 13. The scan time was 2:28 minutes, and the resultant B1+rms was 1.0uT. To review the spine, the series was repeated with a total of 6 slices.
Summary
Adjusting scan parameters to meet the DBS conditions to ensure patient safety and limited RF exposure is possible, but resulted in a modified protocol and reduction in overall qualtiy. The process can be time consuming to ensure that each sequence meets the requirements, however once the protocol is developed it can be repeated and is not patient dependant. Creating protocols for other body parts has proven more challenging. The creation of the checklist assists the technologists in ensuring that all safety steps are performed, as well as validating the B1+rms for each sequence and total RF deposition by scan time. In addition, the form provides are formal documentation that can be added to the patient’s permanent record.Acknowledgements
No acknowledgement found.References
Thorton, John S. (2017). Technical challenges and Safety of magnetic Resonance Imaging with in situ Neuromodulation from Spine to Brain. Official Journal of the European Paediatric Neurology Society, 21, 232-241 Zrinzo,Ludvic. Yoshid, Fumiaki. Hariz,Marwan I. Thorton,John. Foltynie,Thomas. Yousry,Tarek A. Limousin, Patricia. (2011) Clinical Safety of Brain Magnetic Resonance Imaging with Implanted Deep Brain Stimulation Hardware: Large Case Series and Review of Liturature. The Journal World Neurosurgery. 76(1/2), 164-172 Kahan, Joshua. Papadaki, Anastasia. White,Mark. Mancini,Laura. Yousry, Tarek. Zrinzo,Ludvic. Limousin, Patricia. Hariz,Marwan. Foltynie,Tom. Thornton,John. (2015) The safety of Using Body-Transmit MRI in Patients with Implanted Deep Brain Stimulation Devices. PLOS ONE, 10.1371Figures

Figure 1: example of
the checklist utlized during the patient exam
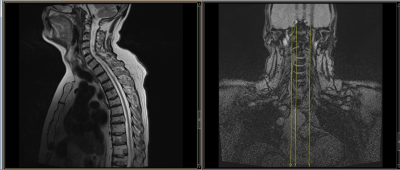
Figure 2: Sagital T2 weighted
imaging and cross reference to meet requirements of less than 2.0uT

Figure 3: Axial T2 weighted
imaging and cross reference to meet requirements of less than 2.0uT

Figure 4: Sagital T2 weighted
imaging and cross reference with 3
slices for requirements of less than 1.2uT
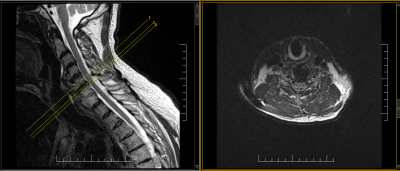
Figure 5: Axial T2
weighted imaging and cross reference with limited slices to meet requirements
for less than 1.2uT