Tracking basal ganglia volume in Parkinson’s disease over 10 years with MRI
1University of Auckland, Auckland, New Zealand, 2Pacific Radiology Group, Christchurch, New Zealand, 3University of Otago, Christchurch, New Zealand, 4The New Zealand Brain Research Institution, Christchurch, New Zealand, 5Monash University, Melbourne, Australia, 6The Department of Electrical and Computer Engineering, University of Canterbury, Christchurch, New Zealand
Synopsis
Basal ganglia degeneration is a hallmark of Parkinson’s disease. However, invivo tracking of these structures has not been fully explored longitudinally.This longitudinal study investigates changes in the volume of basal ganglia nuclei in PD over time, with correlation of these changes in measures of cognition and laterality of motor impairment. At cross-section, the PD group exhibited volume changes in the basal ganglia, but no evidence of a relationship with laterality of symptom onset. Over time, the PD group showed accelerated atrophy relative to controls in the thalamus, caudate, and putamen.
Background
Parkinson’s disease (PD) is a neuro-degenerative disorder affecting approximately 6 million people globally (Elbaz, 2016). In New Zealand the prevalence of PD is expected to double over the next 25 years (Myall et al., 2017), with concomitant increases in personal, social and economic burden. PD has traditionally been considered a motor disorder with emphasis placed on dysfunction within the basal ganglia, but there has been a shift in the clinical and research space in recognising the significance of cognitive impairment and eventual dementia in PD (Hall & Lewis, 2019). Previous work has shown the relevance of basal ganglia to motor impairment (Tuite, 2015; Kalia, 2018), but these structures may also play an important role in the progression of cognitive impairment (Tropea & Chen-Plotkin, 2018). We therefore investigated the relationship between basal ganglia volumes and measures of both motor and cognitive impairment in PD.Methods
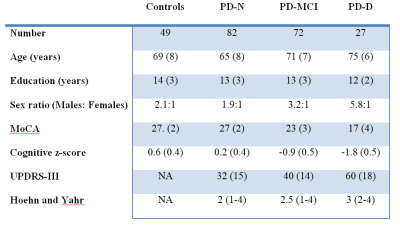
At part of an ongoing longitudinal study, a convenience sample of 181 participants meeting the UK Parkinson’s Disease Society’s criteria for idiopathic PD (mean age (SD) = 69.1 (8.0)) were recruited from the Movement Disorders Clinic at the New Zealand Brain Research Institute (NZBRI), Christchurch, New Zealand; 49 matched, healthy volunteers were also recruited (69.3 (8.4); see table 1). Participants were followed up approximately every two years, up to 10 years. At each time point participants completed comprehensive neuro-psychological testing allowing classification as PD patients with normal cognition (PD-N, n = 82 at baseline), with mild cognitive impairment (PD-MCI, n = 72 at baseline), or with dementia (PD-D, n = 27 at baseline), and a 3T MRI scanning session (GE HDxt, including a 3D T1-weighted spoiled gradient echo sequence: TE/TR = 2.8/6.6 ms, TI = 400 ms, flip angle = 15 deg, acquisition matrix = 256 × 256 × 170, FOV = 250 mm, slice thickness = 1 mm, voxel size = 0.98 × 0.98 × 1.0 mm3).Basal ganglia volumes (thalamus, putamen, caudate, globus pallidus, and accumbens) were calculated using the longitudinal pipeline from Freesurfer (version 6.0.0, https://surfer.nmr.mgh.harvard.edu). Statistical analysis was performed across time and group using Bayesian multi-level regression models to evaluate (1) differences in basal ganglia volumes across cognitive subgroups (Control, PD-N, PD-MCI, and PD-D), (2) whether laterality of motor symptom onset was associated with basal ganglia volume, and (3) whether PD showed accelerated basal ganglia atrophy over time.
Results
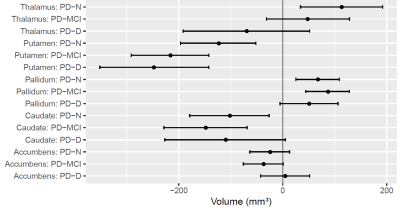
Figure 1 summarises cross sectional volumetric differences across cognitive subgroups. The caudate and putamen showed smaller volumes in all PD subgroups relative to controls, however we identified little evidence of differing amounts of atrophy with increasing cognitive impairment. Interestingly, the pallidum showed increased volumes in PD relative to controls, whereas the thalamus showed a differential pattern of increased volume in PD-N, with subsequent smaller volumes in PD-MCI and PD-D. Over time, the PD group showed accelerated atrophy relative to controls in the thalamus (97% probability), caudate (94% probability), and putamen (84% probability).We found no evidence of an association between side of onset and basal ganglia volume within PD; only pallidum was associated with motor impairment.Conclusions
Similar levels of atrophy in the caudate and putamen across all stages of cognitive impairment in PD are consistent with a general disease-related effect, rather than a cognitive-specific marker. Differential effects within the thalamus (increased volume in PD-N, greatest loss over time) suggest a need for further investigation into this interesting structure. For example, including additional MRI metrics such as diffusion MRI, quantitative susceptibility mapping, and perfusion, as well as cortical metrics, may provide a more complete picture of the process of cognitive decline in PD.Acknowledgements
This work would not have been possible without the support from, and data collected by the New Zealand Brain Research Institute in association with funding and research support from the New Zealand Health Research Council, the Canterbury Medical Research Foundation and Brain Research New Zealand. Further acknowledgement is given to The University of Auckland and the Pacific Radiology Group.References
Elbaz, A., Carcaillon, L., Kab, S., & Moisan, F. (2016). Epidemiology of parkinson's disease. Revue Neurologique, 172(1), 14-26. doi:10.1016/j.neurol.2015.09.0
Hall, J. M., & Lewis, S. J. G. (2019). Neural correlates of cognitive impairment in parkinson's disease: A review of structural MRI findings. International Review of Neurobiology, 144, 1-28. doi:10.1016/bs.irn.2018.09.009
Kalia, L. V. (2018). Biomarkers for cognitive dysfunction in parkinson's disease. Parkinsonism and Related Disorders, 46, S1-S23. doi:10.1016/j.parkreldis.2017.07.023
Myall, D. J., Pitcher, T. L., Pearson, J. F., Dalrymple-Alford, J. C., Anderson, T. J., & MacAskill, M. R. (2017). Parkinson’s in the oldest old: Impact on estimates of future disease burden. Parkinsonism and Related Disorders, 42, 78-84. doi:10.1016/j.parkreldis.2017.06.018
Tropea, T. F., & Chen-Plotkin, A. S. (2018). Unlocking the mystery of biomarkers: A brief introduction, challenges and opportunities in parkinson disease. Parkinsonism and Related Disorders, 46, S1-S18. doi:10.1016/j.parkreldis.2017.07.021
Tuite, P. (2015). Magnetic resonance imaging as a potential biomarker for parkinson's disease. Translational Research, 175, 4-16. doi:10.1016/j.trsl.2015.12.006