What Molecular Properties Can We Image using newly developed X-nuclear MRS methods?
1CMRR, University of Minnesota, Minneapolis, MN, United States
Synopsis
The advancement of ultrahigh-field (UHF) MRI technology (now reaching 10.5T for human scanner and beyond 16T for preclinical animal) has significantly improved imaging sensitivity, spectral and spatiotemporal resolutions. It accelerates new developments of in vivo MRS imaging technologies enabling quantitative and reliable assessment of various neurochemicals, metabolites, metabolic rates in healthy and diseased brain. This lecture will discuss newly developed X-nuclear MRS imaging methods for quantitatively imaging cerebral metabolic rates of glucose and oxygen, ATP production, TCA cycle and NAD redox ratio; and demonstrate promising applications for studying brain function and neuroenergetics under normal and diseased states at UHF.
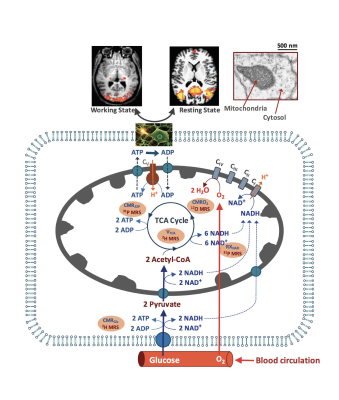
Brain predominantly relies on glucose and oxygen to generate biochemical energy in the form of ATP in mitochondria for supporting electrophysiological activities, neural signaling and brain function under resting and working states. Figure 1 shows a schematic diagram illustrating the complex relations of brain energy metabolism, neuroenergetics, neuro-vascular activity and brain functions. Impaired energy metabolism as well as declined mitochondrial functionality have been hallmarks of various brain disorders including stroke, brain tumor and neurodegenerative diseases. However, there is an unmet need in developing novel neuroimaging tools that can provide sensitive biomarkers to study abnormal physiology and dysfunction in an early stage of brain diseases. Recent progresses in developing multimodal in vivo X-nuclear (e.g., 2H, 17O and 31P) MRSI techniques at UHF have shown great promise for quantitative and noninvasive assessment of fundamental cerebral metabolic rates of glucose (CMRGlc) and oxygen (CMRO2) consumption, ATP production (CMRATP), TCA cycle rate (VTCA) as well as NAD redox ratio (RXNAD) in preclinical animal and human brains (4). In this lecture, we will briefly describe the technical developments and challenges of measuring following physiological parameters of interest using the newly developed X-nuclear MRS imaging methods:
i) quantitative in vivo 2H MRS/MRSI technique for simultaneous measurement of CMRGlc and VTCA (5);
ii) quantitative in vivo 17O MRS/MRSI technique for simultaneous measurement of CMRO2, cerebral blood flow (CBF) and oxygen extraction fraction (OEF) (6-8);
iii) quantitative in vivo 31P MRS/MRSI techniques for measurement of CMRATP and RXNAD (9,10).
We will also discuss and demonstrate new utilities and promise of these X-nuclear metabolic neuroimaging techniques in studying resting-state and stimulus-evoked brain (11-13), stroke (7), brain tumor (14-16), neuroenergetic decline associated with aging (9) and neurodegeneration. These techniques offer new oppotunities to study brain function and disorders, and indicate a potential for translation. Finally, the new imaging methods could be readily employed to other organs beyond brain.
We anticipate that audience with interdisciplinary research background could benefit from this lecture; learn the new MRS imaging techniques about how to employ the techniques for conducting studies, quantify and interpret the in vivo MRS data for addressing biomedical or basic scientific questions; and understand their relevance to clinical application.
Acknowledgements
This work was supported in part by NIH Grants U01 EB026978, R24 MH106049, R01 CA240953, R01 MH111413, RO1 NS057560, RO1 NS070839, P30 NS076408 and P41 EB027061.References
1. Howe FA, Maxwell RJ, Saunders DE, Brown MM, Griffiths JR. Proton spectroscopy in vivo. Magn Reson Q 1993;9(1):31-59.
2. Oz G, Deelchand DK, Wijnen JP, Mlynarik V, Xin L, Mekle R, Noeske R, Scheenen TWJ, Tkac I, Experts' Working Group on Advanced Single Voxel HM. Advanced single voxel 1H magnetic resonance spectroscopy techniques in humans: Experts' consensus recommendations. NMR Biomed 2020:e4236.
3. Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn Reson Imaging 2003;21(10):1263-1281.
4. Zhu XH, Lu M, Chen W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. J Magn Reson 2018;292:155-170.
5. Lu M, Zhu XH, Zhang Y, Mateescu G, Chen W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab 2017;37(11):3518-3530.
6. Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A 2002;99(20):13194-13199.
7. Zhu XH, Chen JM, Tu TW, Chen W, Song SK. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage 2013;64:437-447.
8. Zhu XH, Wiesner HM, Lee BY, Lu M, Chen W. Quantitative and Simultaneous Imaging of CMRO2, CBF and OEF in Resting Human Brain. In: Proc Intl Soc Mag Reson Med; 2015 (oral presentation); Toronto, Canada. p. 895.
9. Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A 2015;112(9):2876-2881.
10. Lu M, Zhu XH, Chen W. In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+ /NADH redox state in human brain at 4 T. NMR Biomed 2016;29(7):1010-1017.
11. Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab 2009;29(1):10-18.
12. Zhu XH, Lee BY, Chen W. Functional energetic responses and individual variance of the human brain revealed by quantitative imaging of adenosine triphosphate production rates. J Cereb Blood Flow Metab 2018;38(6):959-972.
13. Zhu XH, Liu X, Lu M, Wiesner HM, Ugurbil K, Chen W. In Vivo 17O MR Imaging and Quantification of CMRO2, CBF and OEF in Human Visual Cortex at Rest and during Activation. In: Proc Intl Soc Mag Reson Med; 2014; Milan, Italy. p. 3763.
14. Lu M, Zhu XH, Zhang Y, Low W, Chen W. Simultaneous Assessment of Abnormal Glycolysis and Oxidative Metabolisms in Brain Tumor using In Vivo Deuterium 2H MRS Imaging. In: Proc Intl Soc Mag Reson Med; 2016; Singapore. p. 3962.
15. Lu M, Zhu XH, Zhang Y, Low W, Chen W. High-resolution Deuterium MR Spectroscopic Imaging of the Warburg Effect in Brain Tumor. In: Proc Intl Soc Mag Reson Med; 2018; Paris, France. p. Submission.
16. De Feyter HM, Behar KL, Corbin ZA, Fulbright RK, Brown PB, McIntyre S, Nixon TW, Rothman DL, de Graaf RA. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 2018;4(8):eaat7314.