ihMT Principles & Applications
1Aix Marseille Univ, CNRS, CRMBM UMR 7339, Marseille, France
Synopsis
This lecture will cover the basic principles and applications of the recently developed inhomogeneous Magnetization Transfer (ihMT) MRI technique. IhMT is a promising myelin imaging technique and it offers an exciting opportunity to exploit a new endogenous contrast mechanism in vivo using MRI, by discriminating biological tissues based on their dipolar relaxation time (T1D). This presentation will review the basics of the dipolar order concept and associated thermodynamic models. The lecture will also cover typical ihMT experiments and describe up to date MRI sequence optimization. Promising MRI applications will be presented, as well as future research directions.
Target audience
MRI scientists and engineers interested in new contrast mechanisms, Magnetization Transfer techniques, dipolar order imaging, and more specifically inhomogeneous Magnetization Transfer (ihMT). Clinicians interested in myelin imaging techniques.Learning objectives
This lecture will cover the basic principles and applications of the recently developed inhomogeneous Magnetization Transfer (ihMT) MRI technique.Following this lecture, the attendees should:
- understand the origin of dipolar order within spin systems.
- understand how ihMT measure
dipolar order effects and how it is weighted by the dipolar order relaxation
time (T1D).
- know several kinds of RF pulse sequences used for ihMT imaging and their potential for myelin imaging.
Introduction
IhMT imaging is a recent MRI modality that allows indirect observation of dipolar order effects occurring within motion-restricted (semi-solid) macromolecules. It is based on conventional MT, hence requiring magnetization exchange between macromolecules and free water, and the ihMT signal is obtained by combining datasets acquired with specific RF irradiation patterns to isolate the dipolar order contribution to the RF saturation effects occurring within the macromolecular pool. Following the first observations of the ihMT signal (1,2), the contrast mechanism has been elucidated and attributed to dipolar order (3–5), and optimized for various sequence implementations (6–12).IhMT brings a new contrast mechanism to MRI and is intrinsically weighted by the dipolar relaxation time (T1D) (5,9,13), which provides a new way to generate MRI contrast within biological tissues in vivo. While myelin, which has a relatively long T1D component (in the 5-10 ms range (13)) and is readily observable with ihMT, has been the primary application to date (14–20), ihMT may also be adapted to probe shorter T1D components (on the order of or lower than 1 ms) found is other tissues such as skeletal muscle, myocardium, tendon, kidney or liver (9,11,19,21).
This presentation will review the basics of the dipolar order concept and associated thermodynamic models. The lecture will also cover typical ihMT experiments and describe up to date MRI sequence optimization. Promising MRI applications will be presented, as well as future research directions.
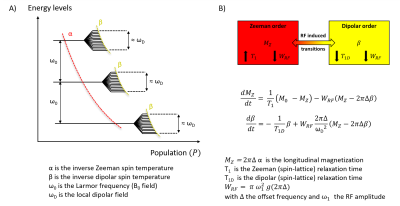
Dipolar order and the spin temperature concept
Motional restrictions occurring in macromolecules lead to broad NRM spectrum due to partial averaging of dipolar couplings and result in additional degrees of freedom within the quantum energy level description. Under the high field, high temperature approximation and following the thermodynamic approach, the spin system may then be described by two independent quasi-equilibrium states corresponding to the usual (Zeeman) longitudinal magnetization MZ, and the inverse (dipolar) spin temperature β (22,23). These states are related to the degree of polarization, or order, of the spins aligned within the static magnetic field B0 (Zeeman order), and with the local dipolar fields ωD (dipolar order). These two independent thermal reservoirs are coupled under RF irradiation according to the Provotorov theory of RF saturation in solids (24,22), which describes thermal mixing for the case of weak RF fields (ω1 << ωD). Combining Bloch equations, to describe the free water pool, and the Provotorov theory to describe the macromolecular pool, one can build a model to interpret and analyze ihMT imaging (3).The ihMT MRI experiment
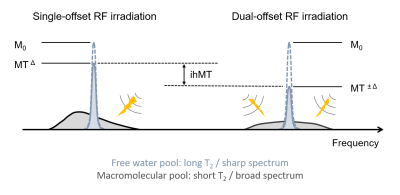
The typical ihMT MRI experiment combines single- and dual-frequency offset RF irradiation to generate a composite image that reflects the intensity of the dipolar order effects occurring within macromolecules. Whereas the single-offset MT experiment is sensitive to dipolar order, the dual-offset experiment is free from dipolar order contribution by symmetry property (3), leading to a signal difference between the two MT experiments that represents the dipolar order contribution to the RF saturation effects.Several ihMT pulse sequences have been developed and may be classified according to the properties of their 1/ MT irradiation pattern (RF power, offset frequencies, timings, simultaneous vs. sequential dual-frequency irradiation, RF duty cycle…), and 2/ associated readout module (2D vs. 3D, single-shot vs. steady state, spoiled GRE vs. SSFP…), or whether these two modules are interleaved or separated in time, such as in magnetization prepared experiments.
As a rule of thumb a given T1D component may be observed with ihMT according to WRFT1D > 0.01 (5) (where WRF represents the RF absorption rate of the macromolecular lineshape), assuming a simultaneous dual-offset saturation is used (i.e. a multiband pulse using a cosine-modulated RF envelope). The frequency alternated approach provides an extra degree of freedom to filter out short-T1D components from the generated signal (9,13). Overall these two mechanisms are driving the T1D-weighting of the sequence and associated specificity for given tissue components such as myelin.
In terms of sensitivity, while the average RF power (constraint by SAR regulatory limitations) and offset frequency are important parameters, it has also been found that low duty cycle RF irradiations, consisting of high intensity RF irradiation phases interleaved with long recovery and mixing periods, provide strong sensitivity enhancement as compared to more distributed RF irradiation patterns (10,11). Of interest low duty cycle experiments also reveal higher signal from short-T1D components, as expected from the higher RF absorption rate, but they may also be associated with short-T1D filtering strategy to recover specificity for long T1Ds, hence leading to a compromise between sensitivity and specificity. The readout module on the other hand contributes to the signal-to-noise ratio and time-efficiency of the technique as for any other MRI application.
In practice, ihMT MRI relies on simple post-processing and has shown good test-retest and multi-site reproducibility (10,25).
Current ihMT applications
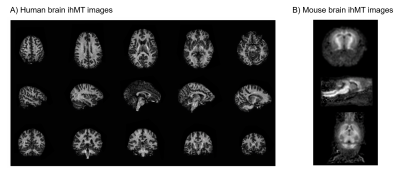
IhMT has been mostly applied in human for central nervous system applications. Several studies have focused on brain or spinal cord examinations in healthy volunteers (e.g. to study brain maturation (20) or cortical myelination (26)) and patients (e.g. MS (16) and ALS (15)), and compared with other myelin sensitive techniques such as conventional MT, myelin water imaging or diffusion MRI (6,14–18,20). Noteworthy rodent applications have also allowed validating the sensitivity of ihMT for myelin by comparison with histology (19).Future research directions
It is naturally expected that more studies will focus on myelin in order to evaluate the added value of ihMT for clinical and neuroscience applications. While other studies will likely focus on other biological targets using ihMT sequences designed to probe shorter T1D components (21), other research directions should aim at a deeper understanding of the ihMT signal and of the contrast mechanisms driving T1D relaxation in biological tissues (e.g. molecular composition, mobility, water accessibility, structural anisotropy…). As illustrative examples, ihMT has been shown sensitive to the white matter fiber orientation (27) and T1D measurements can provide information related to molecular composition and dynamics (28,29).Acknowledgements
I thank all my colleagues, collaborators and other researchers with whom I have had highly stimulating and helpful scientific discussions along this exciting ihMT project.References
1. Alsop DC, de Bazelaire C, Duhamel G. In-vivo Imaging of an Inhomogeneous Component of Magnetization Transfer in Human White Matter. In: Proceedings of the ISMRM. Kyoto, Japan; 2004. p. 2324.
2. Varma G, Duhamel G, de Bazelaire C, Alsop DC. Magnetization Transfer from Inhomogeneously Broadened Lines: A Potential Marker for Myelin. Magnetic Resonance in Medicine 2015;73:614–622 doi: 10.1002/mrm.25174 SMASH.
3. Varma G, Girard OM, Prevost VH, Grant AK, Duhamel G, Alsop DC. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. Journal of Magnetic Resonance 2015;260:67–76 doi: 10.1016/j.jmr.2015.08.024 SMASH.
4. Swanson SD, Malyarenko DI, Fabiilli ML, Welsh RC, Nielsen J-F, Srinivasan A. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magnetic Resonance in Medicine 2017;77:1318–1328 doi: 10.1002/mrm.26210 SMASH.
5. Manning AP, Chang KL, MacKay AL, Michal CA. The physical mechanism of “inhomogeneous” magnetization transfer MRI. Journal of Magnetic Resonance 2017;274:125–136 doi: 10.1016/j.jmr.2016.11.013 SMASH.
6. Girard OM, Prevost VH, Varma G, Cozzone PJ, Alsop DC, Duhamel G. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 2015;73:2111–2121 doi: 10.1002/mrm.25330 SMASH.
7. Prevost VH, Girard OM, Varma G, Alsop DC, Duhamel G. Minimizing the effects of magnetization transfer asymmetry on inhomogeneous magnetization transfer (ihMT) at ultra-high magnetic field (11.75 T). MAGMA 2016;29:699–709 doi: 10.1007/s10334-015-0523-2 SMASH.
8. Girard OM, Callot V, Prevost VH, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Improved imaging strategy for spinal cord applications. Magn. Reson. Med. 2017;77:581–591 doi: 10.1002/mrm.26134 SMASH.
9. Prevost VH, Girard OM, Mchinda S, Varma G, Alsop DC, Duhamel G. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time (T1D) filtering. NMR in Biomedicine 2017;30 doi: 10.1002/nbm.3706 SMASH.
10. Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magnetic Resonance in Medicine 2018;79:2607–2619 doi: 10.1002/mrm.26907 SMASH.
11. Varma G, Girard OM, Mchinda S, et al. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. Journal of Magnetic Resonance 2018;296:60–71 doi: 10.1016/j.jmr.2018.08.004 SMASH.
12. Malik SJ, Teixeira RPAG, West DJ, Wood TC, Hajnal JV. Steady-state imaging with inhomogeneous magnetization transfer contrast using multiband radiofrequency pulses. Magnetic Resonance in Medicine 2020;83:935–949 doi: 10.1002/mrm.27984 SMASH.
13. Varma G, Girard OM, Prevost VH, Grant AK, Duhamel G, Alsop DC. In vivo measurement of a new source of contrast, the dipolar relaxation time, T1D, using a modified inhomogeneous magnetization transfer (ihMT) sequence. Magnetic Resonance in Medicine 2017;78:1362–1372 doi: 10.1002/mrm.26523 SMASH.
14. Taso M, Girard OM, Duhamel G, et al. Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR in Biomedicine 2016;29:817–832 doi: 10.1002/nbm.3530 SMASH.
15. Rasoanandrianina H, Grapperon A-M, Taso M, et al. Region-specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed 2017;30 doi: 10.1002/nbm.3801 SMASH.
16. Van Obberghen E, Mchinda S, le Troter A, et al. Evaluation of the Sensitivity of Inhomogeneous Magnetization Transfer (ihMT) MRI for Multiple Sclerosis. AJNR Am J Neuroradiol 2018;39:634–641 doi: 10.3174/ajnr.A5563 SMASH.
17. Geeraert BL, Lebel RM, Mah AC, et al. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. NeuroImage 2018;182:343–350 doi: 10.1016/j.neuroimage.2017.09.019 SMASH.
18. Ercan E, Varma G, Mädler B, et al. Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magnetic Resonance in Medicine 2018;80:2402–2414 doi: 10.1002/mrm.27211 SMASH.
19. Duhamel G, Prevost VH, Cayre M, et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage 2019;199:289–303 doi: 10.1016/j.neuroimage.2019.05.061 SMASH.
20. Geeraert BL, Lebel RM, Lebel C. A multiparametric analysis of white matter maturation during late childhood and adolescence. Human Brain Mapping 2019;40:4345–4356 doi: 10.1002/hbm.24706 SMASH.
21. Varma G, Callahan C, Girard OM, Duhamel G, Grant AK, Alsop DC. In vivo inhomogeneous magnetization transfer (ihMT) outside the brain using radial ultra-short echo-time acquisitions. In: Proceedings of the ISMRM. Montreal, QC, Canada; 2019. p. p4912.
22. Goldman M. Spin Temperature and Nuclear Magnetic Resonance in Solids. Oxford: University Press.; 1970.
23. Tayurskii D, Le Mehaute A. The Concept of Temperature in the Modern Physics. In: Thermodynamics - Systems in Equilibrium and Non-Equilibrium. IntechOpen. ; 2011. doi: 10.5772/22082 SMASH.
24. Provotorov BN. Magnetic Resonance Saturation in Crystals. Soviet Phys. JETP 1962;14:1126.
25. Zhang L, Chen T, Tian H, et al. Reproducibility of inhomogeneous magnetization transfer (ihMT): A test-retest, multi-site study. Magnetic Resonance Imaging 2019;57:243–249 doi: 10.1016/j.mri.2018.11.010 SMASH.
26. Munsch F, Varma G, Taso M, et al. Myeloarchitectonic mapping of cortical gray matter with 3D inhomogeneous magnetization transfer (ihMT). In: Proceedings of the ISMRM. Montreal, QC, Canada; 2019. p. 1048.
27. Girard OM, Prevost VH, Mchinda S, Varma G, Alsop DC, Duhamel G. Anisotropy of inhomogeneous Magnetization Transfer (ihMT) in White Matter. In: Proceedings of the ISMRM. Honolulu, HI, USA; 2017. p. 472.
28. Lee J-S, Regatte RR, Jerschow A. Magnetization transfer in a partly deuterated lyotropic liquid crystal by single- and dual-frequency RF irradiations. Journal of Magnetic Resonance 2017;281:141–150 doi: 10.1016/j.jmr.2017.05.015 SMASH.
29. Carvalho VND, Hertanu A, Grélard A, et al. MRI assessment of multiple dipolar relaxation time (T1D) components in biological tissues interpreted with a generalized inhomogeneous magnetization transfer (ihMT) model. Journal of Magnetic Resonance 2020;311:106668 doi: 10.1016/j.jmr.2019.106668 SMASH.
Figures