UTE & Other Techniques for Lung MRI
1Guerbet Japan, Japan
Synopsis
Purpose: Pulmonary function tests (PFTs) are global measurements where contributions from regions of normal and varying degrees of alteration in function are combined. Non-uniform disruption of lung architecture is usually assessed by high-resolution computed tomography (CT), which incurs radiation exposure and yields only static anatomical data. Our purpose was to evaluate and define the applications of functional thoracic MRI to bridge anatomical and functional assessment of the lung in regional parenchymal diseases.
Target audiences
Researchers and clinicians interested in regional anatomical and functional imaging of the lung.Outcome/Objectives
Learning the feasibility of proton MRI to assess the anatomy and functions in the lung, one of the most difficult organs, will motivate to develop further applications with exploiting the features of MRI even in the other organs.Purpose
Pulmonary function tests (PFTs) are global measurements where contributions from regions of normal and varying degrees of alteration in function are combined [1]. Non-uniform disruption of lung architecture is usually assessed by high-resolution computed tomography (CT), which incurs radiation exposure and yields only static anatomical data. Our purpose was to evaluate and define the applications of functional thoracic MRI to bridge anatomical and functional assessment of the lung in regional parenchymal diseases.Methods
The utility of ultra-short TE (UTE) imaging in conjunction with a projection acquisition of the free induction decay could reduce the TE to less than 100 µsec and provide a more inherent MR signal from the lung parenchyma which is usually invisible due to its short T2* in conventional MRI methods. With this method at 3T, we first measured signal intensity (SI) and T2* of the normal murine lung parenchyma at different positive end-expiratory pressures (PEEPs). Adjustment of PEEP levels enables the generation of a pseudo-pathological condition in which changes in intrinsic interstitial tissue density can be introduced in a controlled fashion [2]. Subsequently, we measured the SI and T2* in the emphysematous lungs, a real pathology, using a transgenic mouse model [3]. We hypothesized that the capability of the method to acquire inherent MR signal of the lung parenchyma should allow us to assess changes in SI due to inhalation of molecular oxygen or intravenous injection of gadolinium (Gd). Using an animal model of pulmonary embolism (PE), we tested the feasibility of a UTE imaging for assessment of regional pulmonary ventilation/perfusion that are essential for the evaluation of a variety of lung diseases [4]. These applications were expanded to clinical study to investigate the feasibility and utility of the methods in normal subjects and patients in several lung diseases with compared to the results in PFT [5].Results
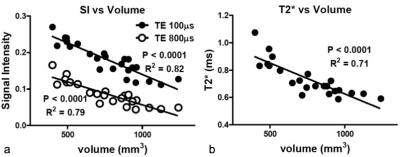
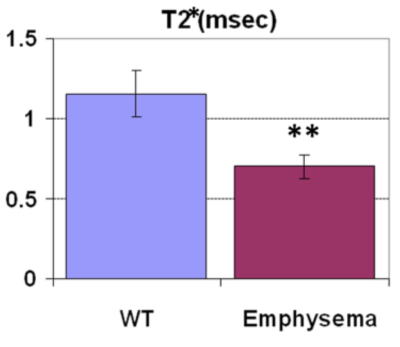
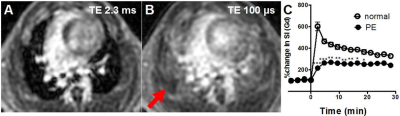
When the lung inflated, the tissue density in the lung was reduced due to enlargement of alveolar airspace, in proportion to reciprocal of increase of lung volume, resulted in reduction of the SI and T2* (Fig. 1). The changing rates in our measured SIs and T2* were larger than that in the estimated alveolar airspace, indicating that the UTE imaging would have the potential to sensitively assess interstitial tissues’ density/volume for the detection and characterization of non-uniform disruption of lung architecture. Further, the emphysematous lung parenchyma had reduced SI and the T2* of the lung parenchyma (0.70 ± 0.07 msec) compared with that in the wild type (1.16 ± 0.15 msec, Fig. 2), which closely related to the parenchymal tissue density (alveolar airspace). Regarding the ventilation imaging by inhalation of 100% oxygen, the T1 of the lung parenchyma was reduced by ~24% (air: 1370 ± 159 ms v.s. 100% oxygen: 1049 ± 93 ms) and led increased SI. The normal parenchymal SI increased 580 ± 172 % right after Gd-injection, and then gradually decreased over time. In the animals that had regional PE, several regions showed perfusion deficit (red allow, Fig. 3) in which the increase of SI was lower up to 15 min after Gd injection than that in the normal parenchyma. By contrast, the abnormal regions showed almost identical changes in SI with the surrounding normal parenchyma in response to oxygen inhalation, indicating ventilation/perfusion mismatch.Discussion
In the sequence, the readout starts immediately after the RF system is switched from transmit to receive so that the MR signal could be acquired before it decays even in the lung parenchyma. The signal is acquired using a center-out sampling scheme, which corresponds to sampling the FID. A minimal duration of the sampling window is archived using radial sampling where the center of k-space is heavily oversampled. These have contributed also to reduce motion artifacts on the UTE images, providing accurate measure of both SI and T2* by reduced responding to inflation of the lung. Further, the high correlations between the SI and T2* and the lung volume suggest that these MRI parameters are sensitive to the tissue density in the lung parenchyma. We postulate that the amount of signal would reflect interstitial tissue density (sum of blood volume, elastic fibers in the alveolar walls and around the blood vessels, bronchi and surfactant) although short T2* effect still reduces the MRI signal, particular in emphysema. UTE imaging was a great advantage in generation of the calculated images (e.g. ventilation/perfusion maps) and assessment of regional parenchymal functions since the currently presented ventilation/perfusion MR imaging methods are particularly sensitive to motion artifact and position mismatch.Conclusion
These methods by UTE imaging have the potential to significantly broaden the scope of state-of-the-art diagnostic imaging capabilities without incurring the risks of radiation exposure. The method is feasible in standard clinical MRI systems.To challenge MRI of the lung, one of the most difficult organs, will advantage you to level up your skills and understandings of MRI and, thus, can help you to solve many issues you may face in the future even in any kind of MRI studies.
Acknowledgements
No acknowledgement found.References
1. J.L. Clausen. The diagnosis of emphysema, chronic bronchitis, and asthma. Clin Chest Med 11:405 (1990).
2. O. Togao, R. Tsuji, Y. Ohno, et al. Ultra-short echo time (UTE) MRI of the lung: Assessment of tissue density in the lung parenchyma. MRM 64: 1491 (2010)
3. M. Takahashi, O. Togao, M. Obara, et al. Ultra-short echo time (UTE) MR imaging of the lung: Comparison between normal and emphysematous lungs in mutant mice. JMRI 32:326 (2010)
4. O. Togao, Y. Ohno, I. Dimitrov, et al. Ventilation/Perfusion imaging of the lung using ultra-short echo time (UTE) MRI in an animal model of pulmonary embolism. JMRI 34:539 (2011)
5. Y. Ohno, H. Koyama, K. Matsumoto, et al. T2* Measurements of 3-T MRI with ultra-short TE: Capabilities of pulmonary functional assessment and clinical stage classification in smokers. AJR 197:w279 (2011)
Figures