Ali Barandov1
1Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
A
new class of non-gadolinium cell-permeable MRI contrast agents have been
developed for monitoring intracellular analytes and processes at the molecular
level. In this talk, we discuss the design, synthesis and applications of such
probes for acquiring spatially resolved functional images of fluctuations in
concentrations of specific analytes in the brains of living subjects. By
improving the technology with more sensitive contrast agents and better brain
delivery strategies, it will be possible to measure and map an expanding array
of neurophysiological processes in animals and ultimately in humans.
Conclusion.
We
have discussed three new classes of non-gadolinium contrast agents based on the
manganese-PDA platform. We also demonstrated the first applications of the contrast
agents in living cells and animals for monitoring biological processes at the
molecular level. Further development of these contrast agents and their
application to functional imaging in living subjects is the focus of our
ongoing research.
Acknowledgements
Acknowledgements
Funding
came from the MIT Simons Center and NIH grants R21-MH102470 and U01-NS090451 to
A.J
References
References
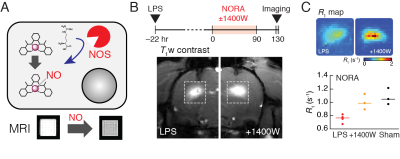
1. A. Barandov,
S. Ghosh, N. Li, B. B. Bartelle, J. I. Daher, M. L. Pegis, H. Collins, A.
Jasanoff, Molecular Magnetic Resonance Imaging of Nitric Oxide in Biological
Systems. ACS Sensors (2020), doi:10.1021/acssensors.0c00322.
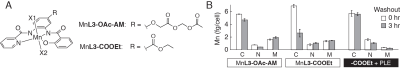
2. A. Barandov, B. B.
Bartelle, B. A. Gonzalez, W. L. White, S. J. Lippard, A. Jasanoff,
Membrane-Permeable Mn(III) Complexes for Molecular Magnetic Resonance Imaging
of Intracellular Targets. J. Am. Chem. Soc. 138, 5483–5486
(2016).
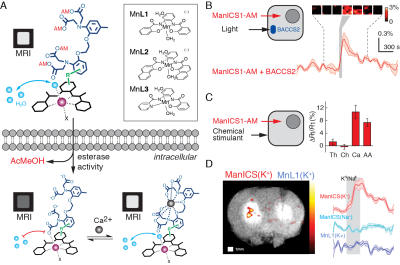
3. A. Barandov, B. B. Bartelle,
C. G. Williamson, E. S. Loucks, S. J. Lippard, A. Jasanoff, Sensing
intracellular calcium ions using a manganese-based MRI contrast agent. Nat.
Commun. 10, 897 (2019).