Cerebellum Development, Pathways & Imaging
1Spinoza Centre for Neuroimaging, Netherlands
Synopsis
The cerebellum is an important, but somewhat overlooked brain region. This lecture will discuss the development and connectivity of the cerebellum, as well as discussing the available imaging tools ready to use to best visualise this beautiful brain structure.
Introduction
The cerebellum, or little brain, is positioned behind the brainstem, inferior to the occipital lobe. Anatomically, it resembles a small brain; it has a white matter tree, also known as the arbor vitae, which contains the iron-rich deep gray matter nuclei and is surrounded by a thin layer of gray matter, the cortex. The cerebellar cortex is much thinner, approximately 1mm, than the cerebral cortex, which can be up to 4 mm thick. It contains three layers of different cell types, as opposed to the 6 known layers in the neo cortex. The deepest cerebellar cortical layer, the granular layer, contains a huge number of neurons, so many that the cerebellum as a whole contains 80% of all neurons in the brain (Herculano-Houzel 2012). This layer is separated from the superficial molecular layer by a thin band of Purkinje cells.The cerebellum is involved in a very wide range of tasks, anything from riding a bike to controlling our emotions. Several neurological diseases cause cerebellar damage, some specific to the cerebellum, such as spinocerebellar ataxia, some more general, such as multiple sclerosis.
Anatomically, the cerebellar cortex is usually described in terms of lobes and lobules separated by fissures. This, however, does not reflect its functional organisation, its connectivity or its development, which follows the parasagittal striping pattern established by different types of Purkinji cells (See Figure 1).
Development
In development, the cerebellum arises from a specialized region at the midbrain/hindbrain boundary (Hallonet 1997). It starts from two separate and symmetric bulges that fuse together to form the cerebellar plate, comprising the vermis (medially) and two lateral hemispheres.During the early development phase, GABAergic neurons (Purkinje cells, olivary projection neurons from the deep cerebellar nuclei and all inhibitory interneurons) originate from the ventricular zone, while all glutamatergic cells (large projection neurons of DCN, unipolar brush cells and granule cells) grow out of the rhombic lip. Projection neurons are produced by fate-restricted progenitors, specializing early in embryogenesis, while interneurons proliferate much later, acquiring mature phenotypes under the influence of local cues. Purkinje cells form the striped pattern characteristic for the cerebellar cortex early in development.
Pathways
The major source of input to the cerebellum is formed by the climbing fibers that originate from the inferior olive nucleus, a structure inside the medulla. Climbing fibres, which are the axons of neurons called olivocerebellar fibers, enter the cerebellum through the inferior cerebellar peduncle. Each climbing fibre then targets one specific Purkinje cell (See Figure 2). One olivocerebellar fibre can produce multiple climbing fibres, which will target Purkinje cells arranged on a single longitudinal stripe (Gruol et al 2016).Mossy fibres come from MF neurons in the pontine nuclei, also carrying information into the cerebellum, but these have targets organised along the lobules, almost perpendicular to the stripes. The granule cells in the deep layer of the cerebellar cortex are connected to mossy fibers and in their turn contact the Purkinje cells and molecular layer interneurons through parallel fibres. Both climbing fibres and mossy fibres will also project to the deep cerebellar nuclei.
The integration of signals from the climbing and parallel fibres in the Purkinje cells will generate complex spikes, propagating along the Purkinje Cell axon to the dentate nuclei neurons, which, in turn, connect to the prefrontal, temporo-parietal and limbic areas (Schmahmann 2010)
Imaging
In terms of imaging, the single most important problem of the cerebellum is that it is often not included, overlooked or segmented away when analysing data. Especially functional data is considered "wholebrain" even if only the neocortex is covered. Historically, this neglect may have been driven by a lack of spatial resolution, which made the mapping of functional results onto cerebellar anatomical structures challenging. Indeed, with the increasing quality of scanner hardware, we also see an increasing interest in cerebellar function, anatomy and changes in disease. Cerebellar imaging at ultra-high field (7T), where especially high spatial resolutions can be acquired, suffers from a lack of B1, especially in the right posterior lobe, which requires either dielectric pads (Teeuwisse 2012) or parallel imaging systems (Padormo 2016) to recover.Useful cerebellar acquisitions are BOLD-based functional imaging, which has recently yielded a cerebellar functional atlas (King 2019) and ever more detailed functional information (van Es 2019). Diffusion imaging has yielded a lot of useful information about the structural connections between the cerebellum and the pons, brainstem and neocortex (Lehman 2020). Anatomically, as for the neocortex, T1 has been a very useful contrast for segmentation of the gray matter layer and for the identification of lesions in multiple sclerosis (Fartaria 2017). Quantitative Susceptibility Mapping (QSM) has very promising applications in the visualisation of the iron-rich deep nuclei, especially the dentate nucleus and in visualizing changes due to cerebellar ataxias (Deistung 2016). Surprisingly, phase contast or QSM may also be used to identify intra-cortical structures in the cerebellum, which is much more difficult to achieve in the neocortex (Marques 2010 and see Figure 3).
With these relatively new developments, neuroscientists and clinicians now have powerful tools to study this fascinating and beautiful brain region, hopefully leading to many new applications and discoveries in the near future.
Acknowledgements
The author would like to thank Chris de Zeeuw and Aleksandra Badura for kindly sharing teaching materials.References
Deistung A, Stefanescu MR, Ernst TM, Schlamann M, Ladd ME, Reichenbach JR, Timmann D. Structural and Functional Magnetic Resonance Imaging of the Cerebellum: Considerations for Assessing Cerebellar Ataxias. Cerebellum. 2016 Feb;15(1):21-25. doi: 10.1007/s12311-015-0738-9. Review.
van Es DM, van der Zwaag W, Knapen T. Topographic Maps of Visual Space in the Human Cerebellum. Curr Biol. 2019 May 20;29(10):1689-1694.e3. doi: 10.1016/j.cub.2019.04.012.
Fartaria MJ, OʼBrien K, Şorega A, Bonnier G, Roche A, Falkovskiy P, Krueger G, Kober T, Bach Cuadra M, Granziera C. An Ultra-High Field Study of Cerebellar Pathology in Early Relapsing-Remitting Multiple Sclerosis Using MP2RAGE. Invest Radiol. 2017 May;52(5):265-273.
Gruol et al. Essentials of Cerebellum and Cerebellar Disorders - a primer for graduate students. Springer 2016. DOI 10.1007/978-3-319-24551-5
Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109: 10661–10668. doi:10.1073/pnas.1201895109
Hallonet M, Alvarado-Mallart RM. The chick/quail chimeric system: a model for early cerebellar development. Perspect Dev Neurobiol. 1997;5(1):17-31. Review.
King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019 Aug;22(8):1371-1378. doi: 10.1038/s41593-019-0436-x
Lehman VT, Black DF, DeLone DR, Blezek DJ, Kaufmann TJ, Brinjikji W, Welker KM. Current concepts of cross-sectional and functional anatomy of the cerebellum: a pictorial review and atlas. Br J Radiol. 2020 Feb 1;93(1106):20190467. doi: 10.1259/bjr.20190467.
Marques JP, van der Zwaag W, Granziera C, Krueger G, Gruetter R. Cerebellar cortical layers: in vivo visualization with structural high-field-strength MR imaging. Radiology. 2010 Mar;254(3):942-8. doi: 10.1148/radiol.09091136.
Padormo F, Beqiri A, Hajnal JV, Malik SJ. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 2016 Sep;29(9):1145-61. doi: 10.1002/nbm.3313.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010 Sep;20(3):236-60. doi: 10.1007/s11065-010-9142-x. Epub 2010 Sep 7. Review.\
Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magn Reson Med. 2012 May;67(5):1285-93. doi: 10.1002/mrm.23108.
Figures

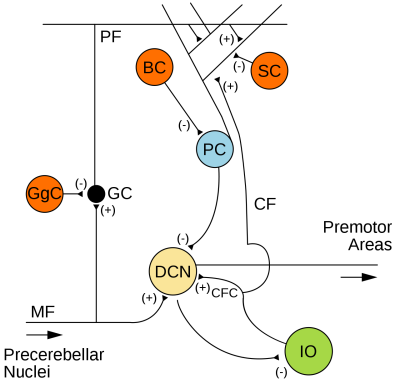
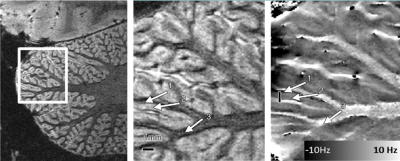
Figure 3. Image highlighting the tight folding pattern of the cerebellar cortex. The phase image in the right panel shows two tissue layers (the granular and molecular layer) within the cerebellar gray matter layer (Figure from Marques 2010)