Probing Microstructure Lengths Scales with Diffusion: Theory
Valerij Kiselev1
1Medical Physics, Dpt. of Radiology, University Medical Center Freiburg, Freiburg, Germany
1Medical Physics, Dpt. of Radiology, University Medical Center Freiburg, Freiburg, Germany
Synopsis
Regimes of diffusion weighting are discussed starting with the simplest measurement with narrow gradient pulses. Such measurements can be classified on a plane of diffusion time and the wave vector induced by the diffusion-sensitizing gradients. Beyond this simple picture are gradients with a finite duration, which radically change the signal behavior for the closed compartment. Versatile diffusion weighting scheme, the successors of the double diffusion encoding, are discussed under the overarching idea of geometry matching between the gradient encoding and the targeted cell population.
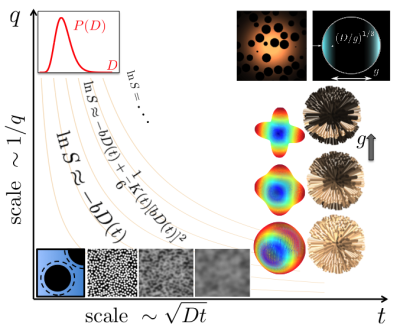
This talk begins as an excursion in the landscape of diffusion weighted signal in the plane formed by the wave vector $$$q$$$ and diffusion time $$$t$$$ [1,2], Fig.1. Limiting the consideration to this plane is a large simplification, only exact in the limit of narrow gradient pulses in which the signal is the Fourier-transformed sample-average diffusion propagator [3]. In the spirit of this relation, a researcher measuring diffusion with a given $$$q$$$ anticipates the sensitivity to the spatial scale $$$1/q$$$, which can be called the default approach. The probed scale will be discussed in more detail below in the context of different measurement techniques. For measurements with long gradient pulses, the sensitivity to the tissue microstructure is specified by the complete time course of a diffusion-weighting gradient $$${\vec g}(t)$$$ with $$$\vec q(t)$$$ being its antiderivative. Both $$$q$$$ and $$$t$$$ in the image should be understood as order-of-magnitude representatives of the detailed function $$$\vec g(t)$$$.
The excursion begins in the corner of small $$$q$$$ and short times. From that point, we see a downslope labelled with the contour lines of the so-called b-value (or b-factor) [4]. These lines show the uncertainty in $$$t$$$ and $$$q$$$, which is essential for measurements in structured media.
We start descending from the highest point in the direction of increasing time keeping the diffusion-weighing gradient small. In this limit, the signal is only sensitive to the diffusion tensor. For short diffusion times, the decrease in $$$D(t)$$$ reveals the total surface of impermeable interfaces in a unit volume of the medium [5]. The question about the scale is answered in the negative form: The measurement is insensitive to the curvature of impermeable interfaces up to small corrections [6]. This technique has been applied to evaluation of the size of prostate lumens [7].
Moving further along the time axis, we note that for a given diffusion time, the typical displacement of molecules is of the order of $$$\sqrt{Dt}$$$. This makes the medium structure increasingly blurred as seen by diffusing molecules, the process called the coarse-graining in physics, which is illustrated with a series of images of a granular two-dimensional medium in Fig.1. It manifests itself in a power-law approach of the diffusion coefficient to its long-time asymptote with the exponent reflecting the effective dimensionality of diffusion and the statistics of long-distance correlations of restrictions on the scale $$$\sqrt{Dt}$$$ [8]. This process has been observed in the normal human brain white matter revealing effectively one-dimensional diffusion [9].
The diffusion length $$$\sqrt{Dt}$$$ is the ``native" scale of a measurement, which means that the signal is sensitive to this scale without any additional suppression. For an NMR measurement, we need values of $$$q$$$ inversely proportional to the coarse-graining length, $$$\sqrt{Dt}$$$. From the practical point of view, this requirement implies a noticeable, but not too strong signal attenuation when $$$bD \sim Dtq^2 \sim 1$$$.
Turning up to go along the $$$q$$$ axis leads further downhill, according to the signal reduction for stronger gradients. By descending from the origin along the $$$q$$$ axis, we come to the domain of short times and strong gradients. In this regime, the signal is simply $$$\langle \exp(-bD) \rangle$$$ where the averaging is performed over the distribution of $$$D$$$ in the sample [10]. The signal does not refer to a definite spatial scale, but one has to realize that $$$D$$$ in the above expression is already averaged on all scales below $$$\sqrt{Dt}$$$ and this length should be much smaller than any other characteristic length in the medium.
Moving parallel to the $$$q$$$ axis for long diffusion times brings us in the park of currently popular models of brain white matter as multiple Gaussian compartments [11,2]. The biological compartments are nearly Gaussian when $$$\sqrt{Dt} \gg \ell_c$$$, where $$$\ell_c$$$ is the correlation length of diffusion heterogeneity in the medium (e.g. the cell size). The models are illustrated with the glyphs showing the increase in the angular resolution of crossing fibers with increasing $$$q$$$, which are largely used for fiber tracking. As it follows from the cumulant expansion, the terms up to the order $$$q^n$$$ contain contributions of spherical harmonics up to the order $$$\ell = n$$$ [12,13,2]. Another set of images illustrates the model of white matter fibers as straight homogeneous cylinders, which are shown with a common origin to focus on their orientation distribution. With the increasing $$$q$$$, less axons contribute to the powder-averaged signal (light color), which results in the observed signal decrease in proportion to $$$1/\sqrt{b}$$$ [14,15,16,17]. For both examples, the probed orientation distribution does not refer to a specific length. However, diffusion length enters the result implicitly by defining the length of axons over which they should be effectively straight. The axon diameter falls out being too small for typical measurements requiring much larger gradients for its detection [8,16], but can be marginally detected by its contribution in the deviation from the $$$1/\sqrt{b}$$$ signal attenuation [17].
The upper right corner of the map contains a swiss-cheese-like image illustrating a possibility to access the correlation function of a porous medium [5]. Such a medium is shaped by excluded volume of a complex geometry, typically NMR-invisible (e.g. porous rock) filled with an NMR-visible fluid (e.g. water) forming a single connected compartment with complex geometry. The key idea is that the diffusion propagator has the form of a nearly Gaussian envelope with a fine structure imprinted by the excluded volume. The signal acquired in this regime is a convolution of a Gaussian propagator, $$$\exp(-Dtq^2$$$) with the medium correlation function. In this way, we access the scale of the medium correlation length, $$$\ell_c \ll \sqrt{Dt}$$$. The price for that is the suppression of the informative part of the signal by the factor $$$(\ell_c/\sqrt{Dt})^d$$$ in $$$d$$$ spatial dimensions.
The plane shown in Fig.1 cannot encompass all possible measurements. Consider the Stejskal -- Tanner pulse sequence with a finite gradient pulses duration $$$\delta$$$ and the interpulse interval $$$\Delta\geq \delta$$$. The diffusion time is not well defined, but can be estimated as $$$t\sim \Delta$$$. In the following two examples, long $$$\delta\sim t$$$ is crucial for the measurement outcome. Consider an NMR-visible fluid with the diffusivity $$$D_0$$$ confined in a closed compartment with the size $$$a$$$, The true diffusion coefficient defined via the mean squared displacement of molecules is $$$D(t)\sim a^2/t$$$ for long diffusion times, $$$\sqrt{D_0 t}\gg a$$$, independent of $$$D_0$$$. The signal attenuation measured with short gradient pulses is $$$\ln S\sim -q^2a^2$$$, from which the value of $$$D(t)$$$ can be obtained correctly. This changes for long, constantly applied gradient for which the signal attenuation is $$$\ln S \sim -g^2a^4 t/D_0$$$ [18,19]. This is the factor $$$a^2/(D_0 t) \ll 1$$$ smaller than for the narrow gradient pulses if we estimate $$$q\sim gt$$$. The same factor compromises an attempt to find $$$D(t)$$$ via the division by the b-value, $$$b\sim q^2t$$$.
Another example is symbolized in the upper right corner in Fig.1. It is the so-called localization regime [20,21,22]. In this regime, the major signal comes from layers adjacent to impermeable interfaces with the thickness about $$$(D/g)^{1/3} \ll \sqrt{Dt}$$$. In contrast to the previous example, application of long gradient pulses suppresses the signal exponentially as $$$\ln S \sim - (bD)^{1/3}$$$. This signal is much smaller than for the narrow gradient pulses for which $$$S\sim 1/q^2$$$ in three dimensions (the diffusion diffraction, [23]). Strictly speaking, this image does not belong the $$$(q,t)$$$ plane. it is rather a projection from the third direction of final $$$\delta$$$, which is not shown in Fig.1.
Nor can numerous methods of combining gradient pairs be shown in Fig.1. These methods can be loosely considered as successors of the double-diffusion encoding [24,25] (going to the continuum limit for the constantly rotated gradients [26,27,28]). The unifying idea of those methods can be called the ``geometry matching", that is suppressing some compartments to measure more specifically other ones using the difference in their shape. For example, the spherical encoding (or isotropic weighting) is used to suppress the signal from everything except for the small compact cells (the dot compartment) with the aim to evaluate their weight in the total signal [29,30]. Another example is the use of planar weighting to narrow the native orientation distribution of white matter axons and suppress the signal from other cells [31].
Note that exchange between tissue compartments is a serious confounding factor for the presently available microstructure modeling. In some cases, it is slow enough to enable the approximation of non-exchanging compartments, but not always. The next talk is focused on this issue.