4882
Tumor Characteristics of Discordant MRI Imaging and Pathology Following Neoadjuvant Chemotherapy in Non-metastatic Breast Cancer1Radiology, University of California, San Diego, La Jolla, CA, United States, 2University of California, San Diego, La Jolla, CA, United States
Synopsis
For patients with non-metastatic or locally advanced breast cancer, MRI imaging is used to follow response to neoadjuvant chemotherapy (NAC) and determine treatment plan. However, MRI radiological complete response is not always concordant with pathological complete response. In a cohort of patients imaged at our institution with non-metastatic/locally advanced breast cancer, we report that 24% of post-NAC final MRI imaging is discordant with the final pathology report. We also show that many of these MRI false negatives demonstrate residual intermediate to high grade DCIS and contain microcalcifications.
Introduction
For patients with non-metastatic or locally advanced breast cancer, neoadjuvant chemotherapy (NAC) is increasingly employed to predict long-term survival, decrease tumor size[1], and allow possible breast-conserving therapy[2]. Identifying response to treatment on imaging is critical to plan patient management. Accurate prediction of pathological complete response (pCR) might define a subset of patients who either wouldn’t benefit from additional chemotherapy, or who can undergo successful breast-conserving surgery[3]. While dynamic contrast-enhanced MRI (DCE-MRI) is most sensitive in identifying residual disease after NAC compared to physical exam, mammography, and ultrasound[4,5,6], change in histopathological tumor subtype following treatment can impair assessment[2]. DCE-MRI can also overestimate or underestimate tumor size, or inaccurately evaluate persistent enhancement of fibrous stroma[7]. As a result, DCE-MRI has variable sensitivity (63-92%)[3,8,9] and specificity (54-91%)[3,8,9] in determining concordant imaging response with pathological response. We retrospectively evaluated a cohort of early stage/locally advanced breast cancer patients imaged with DCE-MRI to evaluate the characteristics that may lead to discordant MRI imaging and pathology following NAC.Methods
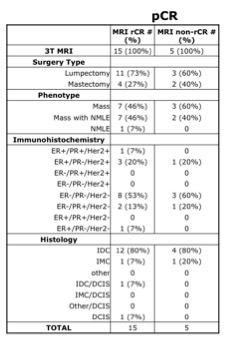
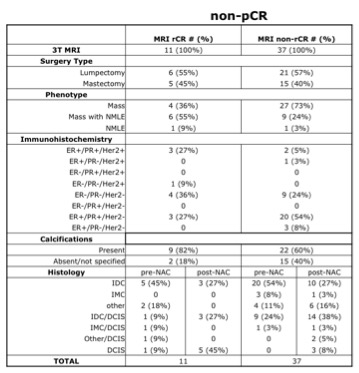
All consecutive patients being treated with NAC for primary invasive breast cancer from August 2015 to September 2019 at our institution, and who underwent DCE-MRI before and after NAC, were included in the study. All breast tumors were evaluated by a fellowship-trained breast radiologist. All patients were imaged in a 3T MRI scanner (MR750, GE Healthcare, Milwaukee, Wisconsin, USA). Post-treatment lumpectomy or mastectomy were performed an average of 29 days following the final DCE-MRI. Based on pathology, patients were classified as having either pathological complete response (pCR) with “no residual disease” on pathology report, or incomplete pathological response (non-pCR) with residual disease identified. Tumors on DCE-MRI reports were retrospectively classified as either rCR (radiological complete response) or non-rCR (radiological incomplete response). Non-rCR included cases of disease progression, no response, or partial response. Tumor histopathology, immunohistochemistry, and phenotype at diagnosis were also evaluated.Results
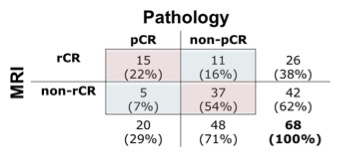
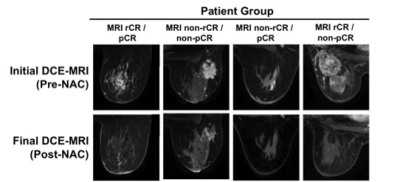
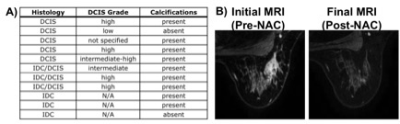
A total of 64 patients (mean age 47, range 20-73 years old) with 68 breast tumors (3 bilateral/1 ipsilateral) were included. Table 1 shows characteristics for patients with pCR and Table 2 shows characteristics for patients with non-pCR. Concurrent findings between final MRI and surgical pathology following NAC were found in 52/68 (76%) tumors. MRI rCR and pCR were in agreement in 22% (15/68) of cases, and non-rCR and non-pCR were in agreement in 54% (37/68) of cases (Figure 1). In our cohort, MRI sensitivity to detect pCR was 75% (15/20), specificity was 77% (37/48), positive predictive value was 58% (15/26), and negative predictive value was 88% (37/42). Figure 2 shows representative cases of breast tumors before and after NAC. Discordance between MRI response and pathology response was identified in 16/68 (24%) tumors. In these lesions, MRI non-rCR corresponded with pCR in 7% (5/68) of cases and MRI rCR corresponded with non-pCR in 16% (11/68) of cases (Figure 1). In the lesions that demonstrated MRI rCR and corresponded with non-pCR, 5/11 (45%) cases showed residual DCIS, while only 3/37 (8%) showed residual DCIS in the concordant MRI non-rCR/non-pCR group. In this discordant group, 4/5 residual DCIS cases demonstrated intermediate to high grade disease and 4/5 residual DCIS cases demonstrated calcifications on final pathology report. Of the 11 lesions demonstrating discordance between final MRI imaging and pathology report, 9 (82%) showed calcifications on pathology report (Figure 3).Discussion
In a cohort of patients with non-metastatic/locally advanced breast cancer undergoing imaging and treatment at our institution, we report a sensitivity of DCE-MRI to detect pCR of 75% and specificity of 77%, which is comparable or slightly higher to previous reports[2,3,8,10]. This may be accounted for by the fact that all of our patients were scanned at 3T, while most prior studies were performed primarily at 1.5T. Post-treatment changes within a tumor including fibrosis, necrosis, or lesion discontinuity can increase or decrease contrast enhancement and make evaluation on MRI challenging[3,9]. While higher histologic grade tumors are more likely to achieve pathologic complete remission[3], MRI is known to be limited in detecting scattered, microscopic tumor areas after NAC[6,11], which can be seen with DCIS[12,13]. Several studies have shown association between residual DCIS on pathology and either Her2 positivity[14], non-mass like enhancement (NMLE) on presentation[10], or discordant MRI imaging following NAC[10]. While our data doesn’t show a clear association between discordant post-NAC MRI imaging and NMLE or Her2 status, we do observe a higher percentage of residual DCIS in the discordant MRI rCR/non-pCR group compared to the concordant MRI non-rCR/non-pCR group. These residual DCIS cases present mostly as intermediate-high grade and contain calcifications. DCIS is frequently composed of linear or branching non-invasive cells, commonly contains microcalcifications, and doesn’t avidly neovascularize compared to invasive disease. Therefore, NAC may not necessarily target DCIS[15,16], and our data are consistent with prior reports that show we are limited in the detection of DCIS on DCE-MRI after NAC[17]. Calcifications were present in 82% of MRI rCR/non-pCR cases in our cohort if residual invasive disease is included (Figure 3). Mammography is extremely sensitive in detecting microcalcifications[17]. To better visualize microcalcifications that may be reflective of residual DCIS, our data may suggest a role for post-NAC mammography in addition to MRI in those women with complete response on MRI.Acknowledgements
NIH EB-RO1000790, UCSD Clinician Scientist Program, GE Healthcare, and California Breast Cancer Research Program Early Career Award.References
1. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001; 30:96-102.
2. Goorts B, Dreuning K, Houwers J, Kooreman L, Boerma EJ, et al. MRI-based response patterns during neoadjuvant chemotherapy can predict pathological (complete) response in patients with breast cancer. Breast Cancer Research. 2018; 20:34.
3. Yuan Y, Chen XS, Liu SY, Shen KW. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysis. AJR Women’s Imaging. 2010; 195:260-268.
4. Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013: 4(2):163-75.
5. Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. European Journal of Cancer. 2010; 46(8):1296-316.
6. Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011; 378(9805):1804-1811.
7. Kim MJ, Kim D, Jung W, Koo JS. Histological analysis of benign breast imaging reporting and data system categories 4c and 5 breast lesions in imaging study. Yonsei Med J. 2012; 53(6): 1203-1210.
8. Gampenrieder SP, Peer A, Weismann C, Meissnitzer M, Rinnerthaler G, et al. Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathological complete response (pCR). Breast Cancer Research. 2019; 21:19.
9. Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013; 105:321-333.
10. Park S, Yoon JH, Sohn J, Park HS, Moon HJ, et al. Magnetic resonance imaging after completion of neoadjuvant chemotherapy can accurately discriminate between no residual carcinoma and residual ductal carcinoma in situ in patients with triple negative breast cancer. PLOS One. 2016; 11:2.
11. Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 2009; 22(1):28-39.
12. Mossa-Basha M, Fundaro GM, Shah BA, Ali S, Pantelic MV. Ductal carcinoma in situ of the breast: MR imaging findings with histopathologic correlation. Radiographics. 2010; 30(6): 1673-87.
13. Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989; 170(2):411-5.
14. Choi HK, Cho N, Moon WK, Im SA, Han W, et al. Magnetic resonance imaging evaluation of residual ductal carcinoma in situ following preoperative chemotherapy in breast cancer patients. European Journal of Radiology. 2012; 81(4):737-43.
15. Kuhl CK. MRI of breast tumors. Eur Radiol. 2000; 10:46-58.
16. von Minckwitz G, Darb-Esfahani S, Loibl S, Huober J, Tesch H, et al. Responsiveness of adjacent ductal carcinoma in situ and changes in Her2 status after neoadjuvant chemotherapy/trastuzumab treatment in early breast cancer—results from the GeparQuattro study (GBG 40). Breast Cancer Res Treat. 2012; 132(3): 863-70.
17. Bazzocchi M, Zuiani C, Panizza P, Del Frate C, Soldano F et al. Contrast-enhanced breast MRI in patients with suspicious microcalcifications on mammography: results of a multicenter trial. AJR. 2006; 186:1723-32.
Figures