4880
Prediction of pathologic complete response of neoadjuvant chemotherapy for early triple negative breast cancer using pre-therapeutic MRI1Univ Lyon, INSA‐Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS UMR 5220, U1206, F69621, Lyon, France, 2Department of Radiology, Centre Léon Bérard, Lyon, France, 3Department of Medical Oncology, Centre Léon Bérard, Lyon, France, 4Department of Pathology, Centre Léon Bérard, Lyon, France
Synopsis
A total of 76 patients were enrolled in this retrospective monocentric study. All patients had a non-metastatic triple negative breast cancer and underwent a pre-therapeutic MRI protocol (T1-weighted, T2-weigthed, diffusion-weighted and dynamic-contrast-enhanced imaging) before a neoadjuvant chemotherapy. Results of radiomic analyses based on multiple contrast images and using 3 classifiers (support vector machine, Random forest and multilayer perceptron) were leading to a relative interest of using a combination of features extracted from DCE-MRI, T1W and T2W in the aim to predict responders and non-responders.
Introduction
Breast cancer is the female cancer with the highest worldwide impact (2,090,000 new cases and 627,000 deaths in 20181). Among the different types of breast cancer, triple negative (TN) cancer is defined by an estrogen and progesterone receptors level lower than 10%, and an absence of over-expression of HER-2 (Human Epidermal Growth Ractor Receptor-2). They represent 10 to 24% of all breast cancers2. TN tumors have a tendency to be bigger, with a highest grade at diagnosis, and with a worse prognosis3. They currently have not targeted therapy yet. Nowadays, neoadjuvant chemotherapy (NAC) treatment are used: i) to reduce the initial tumoral volume in order to allow a conservative surgical treatment; ii) to better eradicate the micrometastatic disease; iii) to test the tumoral chemo-sensibility in order to adjust the choice of further treatments4. The ability to identify responder (with a pathologic complete response: pCR) versus non-responders would enable the use of alternative, potentially more effective therapies. Thus, we investigated the ability of radiomic analyses to predict pCR of TN breast cancer to NAC using pre-therapeutic MRI protocol.Methods
Study designA total of 76 patients were enrolled in this retrospective monocentric study. All patients had an early TN breast cancer and were treated with NAC (anthracyclines-cyclophosphamide then taxanes) before a surgical treatment. They underwent a pre-therapeutic MRI protocol before starting NAC treatment. This study was approved by our institutional review board.
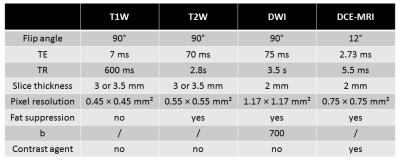
Pre-therapeutic MRI protocol
All breast MR examinations were performed at our institution (Centre Léon Berard, Lyon, France) using a 1.5T Achieva Philips system (Philips Healthcare, Best, The Netherlands) and a dedicated breast surface coil with the patient in a prone position. The pre-therapeutic MRI protocol was included T1-weighted, T2-weigthed, diffusion-weighted and dynamic contrast enhanced imaging (parameters summarized in table 1). Images noted SUB3 were obtained by the subtraction of images acquired 3 min after the injection of gadolinium from the ones acquired before injection (from DCE-MRI).
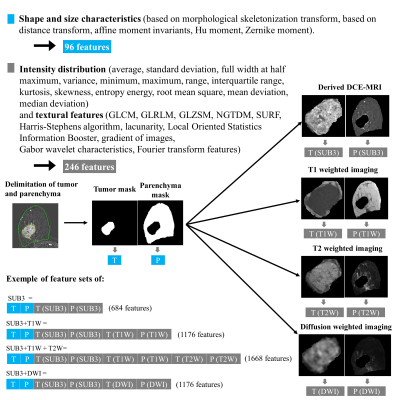
Segmentation and features extraction
The volume of interest (VOI) was delineated manually by an experimented radiologist using itk-SNAP software (www.itksnap.org) on few slices of the SUB3 images and the inter-slice interpolation option was used to complete the mask. Each tumor was segmented individually. VOIs segmented on SUB3 images were used for the extraction of size, shape features and then repositioned on the other images (T1W, T2W and DWI) for texture features (figure1) with home-made software developed on MATLAB-2019a. Multiple configurations of feature set were tested as described in figure 1 to analyze the contribution of multiple contrast images.
Data mining
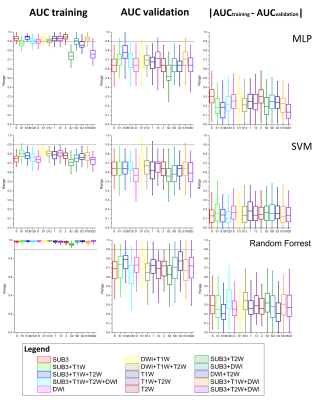
The test set included 25% of the total number of tumors, randomly selected from the whole data set, with a balance between pCR and non-pCR. One hundred different configurations of training set/test set were used. Z-score normalization was applied on each features of the feature set. A dimension reduction was applied using ReliefF method5 to select the twenty most relevant features from the initial data set. From the reduced feature set, supervised machine learning was used to build the prediction model. Three classifiers were evaluated: a multilayer perceptron (MLP) trained with a stochastic gradient algorithm using an adaptive learning rate and a regularization of the synaptic weigths (l = 0.1, 5 mini-batches, 30 hidden nodes, and 60 epochs); a support vector machine (SVM) with a linear kernel; and a random forest (3 splits and 50 learning cycles). The diagnostic performances of the models were evaluated thanks to the area under the curve ROC (AUC) from the test set. AUC difference between the training set and the test sets was used as an indicator of model generalization.
Results
Out of a total of 94 tumors segmented on 76 patients, 57 tumors were imaged with the complete pre-therapeutic MRI protocol. First observation, the reduction dimension method leaded to select principally textural features especially ones provided by the Fourier transform analyses. The figure 2 shows that the random forest classifier had the highest AUC for the training set (mean of 0.97) but the difference between the AUC of training set and the AUC of testing set was slightly higher than the SVM and MLP classifiers leading to more overfitting. The SVM classifier showed the smallest difference of AUC between training set and test set.Discussion/Conclusion
With the present data, SVM appeared to be the best to provide a generalizable predictive model but with a bias on AUC. Random forest and MLP classifiers showed an interest in adding information from multiple contrast imaging (the features extracted from SUB3, T1W and T2W leaded to the best predictive model). Unfortunately, the diagnostic performances depended on the repartition of the data in the training and test sets leading to a large range of AUCs. However, mean AUC values were consistent with the literature6–8 and we were able to find a predictive model with an AUC over 0.90. To conclude, our results shown (i) that the choice of classifier and data repartition in the internal cross-validation procedure may impact model generalization and diagnostic performances; (ii) it possible to predict the pCR of TN breast cancer using radiomic analyses on pre-therapeutic multiple contrast images. Now, this predictive model should be tested on external validation data.Acknowledgements
This work has been funded by LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program "Investissements d'Avenir" (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR) and carried out within the framework of France Life Imaging (ANR-11-INBS-0006). We also acknowledge the SIRIC LyriCAN grant (INCa_INSERM_DGOS_12563).
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953.
2. Billar JAY, Dueck AC, Stucky C-CH, et al. Triple-Negative Breast Cancers: Unique Clinical Presentations and Outcomes. Ann Surg Oncol. 2010;17(3):384-390.
3. Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. N Engl J Med. 2010;363(20):1938-1948.
4. Derks MGM, Velde CJH van de. Neoadjuvant chemotherapy in breast cancer: more than just downsizing. Lancet Oncol. 2018;19(1):2-3.
5. Kononenko I, Simec E, Sikonja MR-. Overcoming the myopia of inductive learning algorithms with RELIEFF. Appl Intell. 1997;7:39–55.
6. Liu Z, Li Z, Qu J, et al. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin Cancer Res. 2019;25(12):3538-3547.
7. Valdora F, Houssami N, Rossi F, et al. Rapid review: radiomics and breast cancer. Breast Cancer Res Treat. 2018;169(2):217-229.
8. Braman NM, Etesami M, Prasanna P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017;19(1):57.
Figures