4869
Prediction of response to neoadjuvant chemotherapy for nasopharyngeal carcinoma using pretreatment MRI: a validated radiomics nomogram1Yuebei People’s Hospital, Shaoguan, China, 2Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 3Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 4Radiology, Stony Brook Medicine, Stony Brook, NY, United States, 5Psychiatry, Stony Brook Medicine, Stony Brook, NY, United States
Synopsis
Nomograms are widely adopted as prognostic tools for clinical practice. In this study, we developed and temporally validated a radiomics nomogram to predict early response to neoadjuvant chemotherapy among patients with nasopharyngeal carcinoma using pretreatment MRI.
Introduction
Nasopharyngeal carcinoma (NPC) is prevalent throughout east and southeast Asia, accounting for >70% of 129,000 new cases worldwide in 2018 although it is relatively uncommon comparing to other cancers[1]. Neoadjuvant chemotherapy is recommended by the National Comprehensive Cancer Network (NCCN) Guidelines to improve survival rates. However, such treatment can cause side effects, which reduce quality of life. Additionally, some patients do not respond to chemotherapy and thus are subject to a toxic treatment without clinical benefit. A previous study[2] demonstrated the capability of MRI quantitative imaging biomarkers for response prediction. However, limitations of their study included the use of a 2D ROI, and not incorporating clinical characteristics into the model, and a validated nomogram for clinical practice is still lacking. Therefore, we aimed to develop and validate a radiomics nomogram to predict the response to cisplatin-based neoadjuvant chemotherapy in NPC patients using pretreatment contrast-enhanced T1-weighted and T2-weighted MRI. The nomogram was validated using a temporal validation set.Materials and Methods
A total of 105 NPC patients were enrolled in this prospective study approved by local IRB with written consent obtained. Among all patients who received cisplatin-based neoadjuvant chemotherapy, 63 patients were confirmed to have response to neoadjuvant chemotherapy according to RECIST guideline[3]. All patients underwent non-contrast and contrast-enhanced nasopharyngeal and neck scans on a 1.5 T MR scanner (Signa EXCITE HD, TwinSpeed, GE Healthcare, Milwaukee, WI, USA). Regions-of-interest (ROI) covering the whole tumor were manually delineated by a radiologist using ITK-SNAP, and a total of 850 radiomic features were extracted from each ROI using Matlab 2018a and LIFEx[4], including shape, first-order, texture, wavelet and Laws features. The dataset was split into a training cohort (n=70 with 42 positively responding to chemotherapy, before August 2018) and a temporal validation cohort (n=35 with 21 positively responding to chemotherapy, after September 2018). To avoid overfitting and bias, training cohort was oversampled and temporal validation cohort was undersampled. Features with strong discriminative power were chosen by pre-selection (Mann Whitney U-test p<0.05, Spearman correlation |r|<0.7) followed by least absolute shrinkage and selection operator (LASSO) and logistic regression with 10-fold cross-validation to develop a clinical-radiomics nomogram in the training cohort. The receiver operating characteristic (ROC) analysis was performed with the optimal threshold determined by maximizing the Youden index to assess the model performance.Results and Discussion
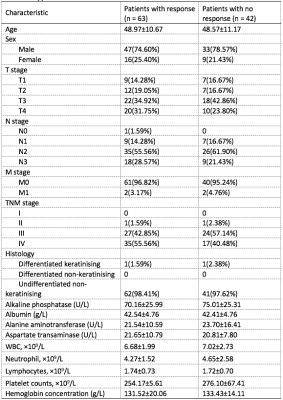
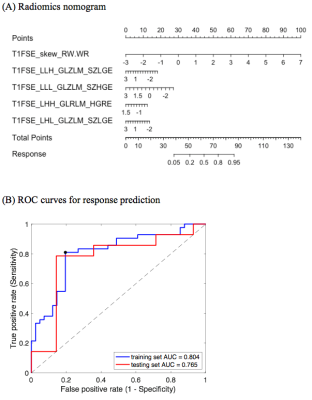
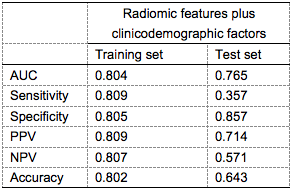
Clinicodemographic characteristics for patients with and without response are depicted in Table 1. Using the training cohort, five radiomic features were selected for the prediction model, with area under the ROC curve 0.804. The corresponding temporal validation AUC was 0.765 (Figure 1B, Table 2). The nomogram developed from the radiomics signature is shown in Figure 1A. Pretreatment radiomics nomogram could aid in clinical decision making and benefit NPC patients, especially for non-responders, helping them avoid the serious side effects and receive radiation therapy at an earlier time. Cross-scanner and cross-center reliability will need to be further validated.Conclusion
A radiomics nomogram has been developed and temporally validated for predicting response to neoadjuvant chemotherapy for NPC patients based on routine pretreatment MRI.Acknowledgements
No acknowledgement found.References
1. Chen, Y.-P., et al., Nasopharyngeal carcinoma. The Lancet, 2019. 394(10192): p. 64-80.
2. Wang, G., et al., Pretreatment MR imaging radiomics signatures for response prediction to induction chemotherapy in patients with nasopharyngeal carcinoma. Eur J Radiol, 2018. 98: p. 100-106.
3. Eisenhauer, E.A., et al., New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228-47.
4. Nioche,
C., et al., LIFEx: A Freeware for
Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in
the Characterization of Tumor Heterogeneity. Cancer Res, 2018. 78(16): p. 4786-4789.