4868
Quantify breast stiffness in breast carcinoma patients using Magnetic Resonance Elastography at 3T and its comparison with clinical grading1Radiology, Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
Dynamic contrast-enhanced MRI kinetic parameters have been investigated to assess the response to neoadjuvant chemotherapy (NAC). However, those studies have predictive value only after one or two cycles of NAC treatment and involves injection of contrast agent. Moreover, breast tumor sample is graded clinically using biopsy and is invasive. Aim of this study is to evaluate any correlation between MRE stiffness and clinical grading in breast carcinoma and if MRE can predict the response to NAC. Preliminary results show moderate correlation between MRE-derived stiffness and clinical grading in breast carcinoma patients.
Purpose
Neoadjuvant chemotherapy (NAC) has gained clinical acceptance in the patients with breast cancer [1, 2]. Dynamic contrast-enhanced MRI (DCE-MRI) kinetic parameters have been investigated to assess the response to NAC [3, 4]. However, those studies have predictive value only after one or two cycles of NAC treatment and in addition involves the injection of contrast agent. In addition, sample of tumor tissue is graded clinically using biopsy and is invasive. Magnetic resonance elastography (MRE) is a non-invasive technique to estimate stiffness of soft tissues and has been used in the breast [5,6]. The objective of this study is to evaluate if there is any correlation between MRE-derived stiffness and clinical grading of breast carcinoma and if MRE can predict the response to NAC as well as the enhancement characteristics (i.e. kinetic parameters) of different tumor types in breast carcinoma patients.Methods
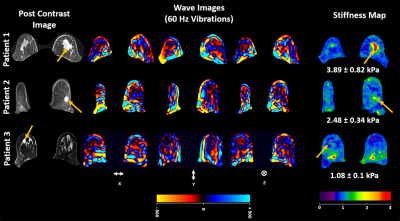
All imaging was performed using a 3T MRI scanner (Skyra, Siemens Healthcare, Erlangen, Germany). Written informed consent was obtained from all volunteers (n=12, age range: 35 to 64 yrs). Axial slices were obtained using a spin-echo Echo planar imaging (SE-EPI) MRE sequence. Experimental setup with patient lying on the breast coil face down and 60 Hz vibration was introduced through a soft sternum driver as shown in figure 1. Imaging parameters included: TR/TE: 833.33/43.6 ms; EPI factor: 128 (4 shots); FOV=320x320mm2, matrix size=256x256, slice thickness=3mm, number of slices=10, MRE phase offsets=4. Motion encoding gradient of 60Hz was applied separately in the x, y and z directions to encode in-plane and through plane displacement fields. MRE images were masked to obtain the breast and a curl processing along with directional filtering with cutoff values of 6 to 30 waves/FOV was performed to remove longitudinal component of motion along with reflected waves. Finally, DI method with the laplacian of Gaussian kernel was performed to obtain weighted stiffness map. Clinical findings for patients such as carcinoma grade score were used and correlated with MRE derived stiffness. 2 of 12 data points were excluded since they were ductal carcinoma in situ (DCIS) and clinical grade was not applicable.Results
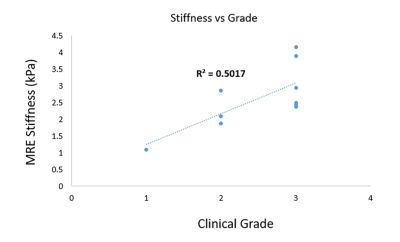
Figure 1 illustrates post contrast image, snapshot of one of the time points of wave propagation in three spatial directions x, y and z and the corresponding 3D weighted stiffness map in 3 breast carcinoma patients.Figure 2 shows linear correlation plot between MRE-derived stiffness and clinical grading in 10 breast carcinoma patients. Moderate correlation (R2=0.50) was found.
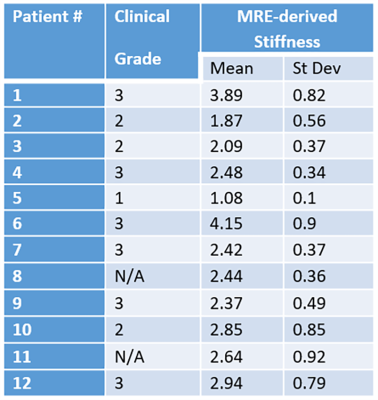
Table 1 shows clinical grade, MRE-derived mean stiffness and standard deviation in each patient.
Conclusion
Preliminary results demonstrates moderate correlation between MRE-derived stiffness and clinical grading of breast carcinoma. Future work will involve more datasets and comparison of MRE-derived stiffness with NAC.Acknowledgements
Funded by NIH R01HL124096.References
1. Loo CE, Teertstra HJ, Rodenhuis S, van de Viver DA, Hannemann J, Muller DA, Peteters MJ, and Gilhuijs KG (2008). Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR Am J Roentgenol 191, 1331–1338.
2. Le-Petross HC and Hylton N (2010). Role of breast MR imaging in neoadjuvant chemotherapy. Magn Reson Imaging Clin N Am 18(2), 249–258.
3. Ah-See MLW, Makris A, Taylor NJ, Harrison M, Richman P, Burcombe R, Stirling J, d’Arcy JA, Collins DJ, and Pittam MR, et al (2008). Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 14(20), 6580–6589.
5. Li X, Arlinghaus L, Ayers GD, Chakravarthy AB, Abramson DA, Abramson VG, Atuegwu N, Farley J, Mayer IA, and Kelley MC, et al (2014). DCE-MRI analysis methods for predicting the response of breast cancer to neoadjuvant chemotherapy: pilot study findings. Magn Reson Med 71(4), 1592–1602. [5] McKnight, Alexia L., et al. "MR elastography of breast cancer: preliminary results." American journal of roentgenology 178.6 (2002): 1411-1417.
6. Sinkus, Ralph, et al. "Viscoelastic shear properties of in vivo breast lesions measured by MR elastography." Magnetic resonance imaging 23.2 (2005): 159-165.
Figures