4867
Radiotherapy for prostate cancer: Effects of fiducial gold marker on diffusion-weighted magnetic resonance imaging
Osamu Tanaka1, Takuya Taniguchi1, Kousei Ono1, and Masayuki Matsuo2
1Radiation Oncology, Asahi University Hospital, Gifu, Japan, 2Radiation Oncology, Gifu University Hospital, Gifu, Japan
1Radiation Oncology, Asahi University Hospital, Gifu, Japan, 2Radiation Oncology, Gifu University Hospital, Gifu, Japan
Synopsis
Gold markers showed little effect on the quality of DWI. Therefore, despite using iron-containing markers and the size of marker < 0.5 mm being available, MRI, particularly DWI, may be used during follow-up imaging.
Introduction:
Precise irradiation is required.
Thus, IMRT has been increasingly performed using fiducial gold markers as means
of CT/MRI fusion. In addition, increasing number of studies have reported that
results of diffusion-weighted imaging (DWI) and apparent diffusion coefficient
(ADC) of MRI are associated with prostate-specific antigen (PSA) in the
assessment of efficacy of RT for prostate cancer.
Meanwhile, when fiducial markers
are placed in the prostate, their size and iron content may affect image
quality. DWI is easily affected by metals, and no study has compared the
quality of DWI before and after fiducial marker placement in the prostate.
Moreover, change in ADC before and after marker placement has not been
evaluated properly. In other words, change in the background of DWI after
marker placement before RT may hinder evaluation. Therefore, we prospectively
assessed effects of fiducial gold marker on DWI during RT for prostate cancer.
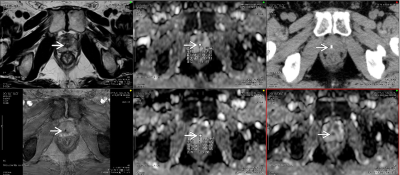
Materials and Methods:
Twenty-one patients in whom two
gold markers were placed on the prostate with abnormal signal intensity on DWI
were evaluated. No patients received hormonal therapy or neoadjuvant
chemotherapy either before or during the course of IMRT. Contouring, prostate volume measurements, and
OAR determination were performed by the same radiation oncologist. MRI was
performed in all patients and two gold fiducial markers were placed in the
prostate 3 weeks before the CT/MRI fusion setting. CT was performed, followed
by MRI within 20 min. MRI was obtained using a five-channel cardiac coil (3-mm
section thickness, with no intersection gap, and 16-cm field of view).
Parameters for DWI were as follows: spin echo with echo planner image (EPI)
[TR/TE in ms]: (2264/70); NSA: 8 times; PES: 103; FES:128; TPR;
frequency/phase: 2.58/3.21; and a diffusion b-factor of 1000 s/mm2.
A radiologist and medical physicist
evaluated each image independently. The following were evaluated: Image quality
on a scale of 1–5: 5 points indicate no change in the quality of DWI before and
after marker placement; 4 points indicate marginally better than 3; 3 points
indicate no effect of signal void on diagnosis; 2 points indicate slightly poor
than 3; and 1 point indicates the lack of evaluation. High score regarded
clinically useful.
Change in ADC (10−3 mm2/s)
before and after gold marker placement (ADC after placement − ADC before
placement). The region of interest (ROI) was the maximum axial cross section of
the area with abnormal signal intensity in the prostate at DWI. Further, gold
makers were placed in the abnormal intensity on DWI. Contouring the ROI was
performed with the same size and location before and after placing the markers
in the prostate. The difference was transformed to an absolute value because
ADC increased or decreased in different cases.
Results:
Mean effect of markers on DWI
measured by the radiation oncologist and medical physicist was 4.3 (Standard
Deviation (SD) 1.3, range 2–5) points and 4.0 (SD 1.4, range 3–5) points,
respectively.
Mean change in ADC calculated by
the radiation oncologist and medical physicist was 0.45 (SD 0.41, range
0.25–0.89) and 0.34 (SD 0.58, range 0.12–0.79), respectively.
Conclusion:
Gold markers showed little effect
on the quality of DWI. Therefore, despite using iron-containing markers and the
size of marker < 0.5 mm being available, MRI, particularly DWI, may be used
during follow-up imaging.