4864
The value of multiparametric MRI-based radiomics in diagnosing of benign and malignant prostatic diseases1Guangdong Medical University, Zhanjiang, China, 2Huiying Medical Technology Co., Ltd., Beijing, China, 3Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Synopsis
Radiomics, as it refers to the extraction and analysis of large number of advanced quantitative radiological features from medical images using high throughput methods, it provides a new evaluation method for detection of the suspicious lesion of cancer. The present study explores the value of radiomics based on the mulitparameter magnetic resonance imaging (mpMRI) for diagnosis of the benign and malignant prostatic diseases.
Introduction
Prostate cancer is one of the most common malignancies of male urinary system, which is similar to the symptoms of prostatic hyperplasia, prostatitis, and low grade epithelial prostate internal tumour in earlier stage. And with the disease progress, it can bring about hematuria. In recent years, with the change of dietary structure, the morbidity and mortality of prostate cancer is gradually increase.1,2 The onset of prostate cancer is hidden, the natural course of disease is relatively long, in the early clinical symptoms are not obvious, diagnosis is difficult, patients with conscious symptoms often progress, or lose the best time of treatment. Therefore, early diagnosis of prostate diseases can not only provide guidance for treatment, but also determine the prognosis, curative effect and survival time of PCa patients.The use of multiparametric MRI (mpMRI) is rapidly gaining momentum in the management of prostate cancer because of its improved diagnostic potential and its ability to combine functional and anatomical information, and it provides important reference evidence for the identification of benign and malignant prostate lesions.3,4 Radiomics is a type of quantitative medical image analysis, which is perfectly suited for multiple series of prostate mpMRI sequences analysis. It has been reported that radiomics have shown great potential in tumor detection and localization as well as prediction of treatment response.5,6 And the purpose of this study was to investigate the effect of mpMRI-based rodiomics on benign and malignant prostate diseases.Materials and methods
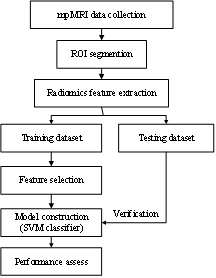
A total of 60 patients with prostate cancer (35) and prostatic hyperplasia (25), who were underwent MRI examination and were confirmed by pathology in Affiliated hospital of Guangdong medical university, were collected retrospectively from Jan 1st, 2016 to Nov 30th, 2018. All patients underwent the mpMRI exam of the prostate typically includes acquisition of T2-weighted (T2WI), diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE)-MRI. The open source software ITK-SNAP is used for 3D tumor segmentation manually, and the region of interest (ROI) was delineated on each slice of axial T2W, DWI and DCE, by two five-years work experienced radiologists according to detailed records of prostate biopsy and pathological diagnosis, which is shown in Figure 1. After the tumor segmentation, a total of 1409 quantitative imaging features were extracted from each mpMRI sequences with Radcloud platform (https://mics.radcloud.cn), which including the shape, signal intensity and texture features. Then, the variance threshold method, select K best methods, and least absolute shrinkage and selection operator (LASSO) algorithm were used to choose the features most significant to the prostate diseases on each single sequence of DWI, T2WI, DCE, and the joint sequences of DWI+T2WI, DWI+DCE, and DWI+T2WI+DCE, respectively. After the subset of useful features has been identified, six radiomics-based models were constructed with the selected features of each single sequences and the joint sequences respectively on the training dataset to classify the patient, malignant versus benign disease. The five-fold cross-validation method and support vector machine (SVM) algorithms were utilized for differentiation model constructing. And the diagnostic performance of the models were verified on the validation dataset, and were evaluated by receiver operating characteristic curves with indicators of sensitivity, specificity. The whole process of the radiomics workflow is shown in Figure 2.Results
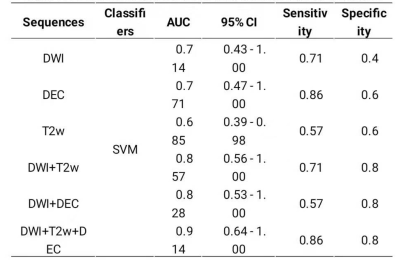
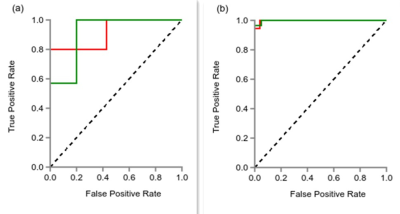
After feature selection by LASSO method, the numbers of remaining features of DWI, T2WI,DEC,and the combined sequences DWI+T2WI, DWI+DCE, and DWI+T2WI+DCE were 17, 11, 17, 16,16, and 22, respectively. The diagnostic performances of the six differential models on validation dataset were shown in Table 1. As we can see, the combination of three sequences (DWI+T2WI+DCE) had the best diagnostic performance with the highest AUC (0.914, 95% CI: 0.64-1.0), sensitivity (0.86), specificity (0.80) on the validation dataset. Of single sequence, the DCE showed better performance with AUC (0.771, 95% CI: 0.47-1.0), sensitivity (0.86), and specificity (0.6) than any others. And Figure 3 showed the ROC curve of the combined sequence DWI+T2WI+DCE on training dataset and validation dataset.Conclusion and discussion
Radiomics models based on multi-parameter magnetic resonance imaging has high potential for prostate diagnosis. Especially, the radiomics model with multi-sequences of multi-parameter MRI has the better performance in diagnosing of benign and malignant prostatic diseases.Acknowledgements
I would like to express my sincere gratitude to my supervisor, professor Zebin Luo, for his meticulous guidance and selfless help during my postgraduate study.The tutor's dedication to work spirit, rigorous and realistic attitude of scholarship, left me a very deep impression, is my future learning example.I sincerely thank director Wubiao Chen of the imaging department for his help and support, and all the teachers in the MRI room, especially Dr. Chen , for his guidance and help in data collection, data analysis and other aspects.Sincerely thank Dr. Dai for his guidance and help in data analysis and other aspects.Thank my family for their understanding and support!Finally, I sincerely thank all the people who helped me once again, thank you.References
1. Fei Yang, John C. Ford, Nesrin Dogan, et al. Magnetic resonance imaging (MRI)-based radiomics for prostate cancer radiotherapy. Translational Andrology and Urology. 2018, 7(3): 445-458
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018, 68: 7-30
3. Toivonen J, Montoya Perez I, Movahedi P, et al. Radiomics and machine learning of multisequencen multiparametric prostate MRI: Towards improved non-invasive prostate cancer characterization. 2019, PLoS ONE 14(7): https://doi.org/10.1371/journal.pone.0217702
4. Bourbonne V, Vallières M, Lucia F, et al. MRI-Derived radiomics to guide post-operative management for high-risk prostate cancer. Frontiers in Oncology. 2019, 9, 807. doi: 10.3389/fonc.2019.00807
5. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012, 48: 441-446.
6. Stoyanova R, Takhar M, Tschudi Y, et al. Prostate cancer radiomics and the promise of radiogenomics. Transl Cancer Res. 2016, 5(4): 432-447
Figures