4852
Dynamic Contrast Enhanced MRI Parametric Maps as Indicators of Lymph Node Radiotherapy Response in Head and Neck Cancer1Radiation Oncology, MD Anderson Cancer Center, Houston, TX, United States, 2Radiation Physics, MD Anderson Cancer Center, Houston, TX, United States, 3Head and Neck Surgery, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Current methodology in analyzing pathological lymph node radiotherapy response in head and neck cancer relies on qualitative visual inspection of geometrical features. A quantitative approach that takes into account dynamic changes in underlying lymph node pathology could be an important supplemental clinical tool in radiotherapy treatment planning. Herein, we investigate dynamic contrast enhanced MRI quantitative parametric maps as possible biomarker candidates for lymph node radiotherapy response by analyzing multi-timepoint images of head and neck cancer patients.
Introduction
Head and neck cancer (HNC) is a devastating disease that affects a large portion of individuals every year. Conventional treatment involves radiotherapy which targets the gross tumor volume (GTV). Malignant cervical lymph nodes are considered as part of the GTV in HNC and viewed as negative prognostic indicators of disease recurrence, distant metastases, and survival rates 1. The development of noninvasive imaging biomarkers for use in treatment planning to detect early nodal response to therapy could have the potential to improve radiotherapy efficacy in HNC such as through dose modification. Dynamic contrast enhanced (DCE)-MRI is a functional imaging technique that has been linked to tissue perfusion and microvascular status through quantitative parametric maps 2 but has only been modestly investigated in lymph nodes with a focus on static comparisons between benign and malignant lesions 3,4 . Therefore, we attempt a proof of concept study to determine if DCE-MRI parametric maps can be candidates for temporal biomarkers of mid-therapy lymph node response.Methods
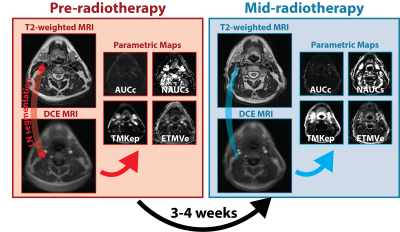
Patients were enrolled under an IRB-approved protocol as part of an ongoing prospective clinical trial. All patients underwent two MRI scans: a baseline pre-therapy scan before beginning radiotherapy and a mid-therapy scan 3-4 weeks after radiotherapy initiation. Complete response (CR) and non-CR were determined through response evaluation criteria in solid tumors (RECIST1.1) criteria 5. Manual segmentations of pathologic lymph nodes receiving irradiation as part of the GTV were performed by a radiologist using T2-weighted MRI anatomical sequences for each time point, co-registered to DCE-MRI sequences, and propagated to respective quantitative DCE parametric maps (Figure 1). Mean values for 11 quantitative (derived from pharmacokinetic models) and semi-quantitative parametric maps including the area under the curve in tissue (AUC), normalized AUC (NAUC), Tofts Model (TM) 6, Extended Tofts Model (ETM) were calculated for each segmented lesion at each timepoint. These maps are referred to as AUCc, AUCs, NAUCc, NAUCs, TMKep, TMKtrans, TMVe, ETMKep, ETMKtrans, ETMVe, and ETMVp. The Wilcoxon signed-rank test was used to assess temporal variation of quantitative parametric maps for all lesions. The Mann-Whitney U test was used to compare lesions that achieved CR at mid-therapy with those that did not. Changes in parametric maps were also associated with volume through a Pearson correlation to determine the relationship between these parameters and a more commonly available clinical metric.Results
A total of 27 patients with 38 lymph node lesions were included in the final analysis. 14 lesions had a CR while 24 lesions had a non-CR. Mid-therapy AUCc, AUCs, and NAUCs were significantly higher than pre-therapy values for all lesions (p<0.005). TMKep, and ETMKep were significantly lower than pre-therapy values for all lesions (p<0.05). Pre-therapy NAUCc, TMKtrans, TMVe, ETMKtrans, ETMVe, and ETMVp were not significantly different than mid-therapy values for all lesions (p>0.05). Changes in AUCc, AUCs, TMKep, and ETMKep were significantly higher for CR lesions when compared to non-CR lesions (p<0.005). Changes in NAUCc, NAUCs, TMVe, and ETMVe were significantly lower for CR lesions when compared to non-CR lesions (p<0.05). Changes in TMKtrans, ETMKtrans, and ETMVp were not significantly different for CR lesions when compared to non-CR lesions (p>0.05). No strong correlations for any of the changes in parametric maps were found for changes in volume, (r = -0.12, -0.28, -0.08, -0.13, 0.35, 0.10, -0.07, 0.25, -0.02, -0.04, -0.01 for AUCc, AUCs, NAUCc, NAUCs, TMKep, TMKtrans, TMVe, ETMKep, ETMKtrans, ETMVe, and ETMVp respectively).Discussion
Herein we have shown that certain quantitative parametric maps derived from DCE-MRI demonstrate significantly measurable changes during radiotherapy and can differentiate lymph nodes with mid-treatment CR or non-CR. These parametric maps have been linked to vascularity and blood flow distribution in previous literature, demonstrating their underlying pathological significance 2. Our results highlight the potential of these parameters to help target therapeutic intervention based on their temporal changes as indicators of response. Moreover, we demonstrate that these parametric maps are not strongly correlated to reductions in volume, a commonly available geometric quantification of tumor response, and therefore may yield novel information into the underlying pathological response of lymph node metastasis when compared to traditional qualitative metrics.Conclusions
We suggest DCE-MRI contains underlying pathological information related to changes in lymph nodes, primarily related to vascularity, and may yield additional or supplementary information for clinician to use in the future for radiation therapy planning. Quantitative imaging such as that achieved by DCE-MRI may be a useful tool to help target personalized medicine therapeutics in oncology by indicating therapeutic response potential of malignant tissues. Building off these preliminary results, we will expand our analysis to a larger cohort of patients with external validation, probe deeper into the underlying pathological significance of these parametric map changes, and utilize parametric maps in predictive modeling of patient clinical outcomes.Acknowledgements
Supported by a training fellowship from The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (Grant No. TL1 TR003169).References
1. Leemans, C. R., Tiwari, R., Nauta, J. J., Waal, I. V. D. & Snow, G. B. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 73, 187-190 (1994).
2. Chikui, T. et al. The principal of dynamic contrast enhanced MRI, the method of pharmacokinetic analysis, and its application in the head and neck region. International journal of dentistry 2012 (2012).
3. Yan, S. et al. Characterization of cervical lymph nodes using DCE-MRI: differentiation between metastases from SCC of head and neck and benign lymph nodes. Clinical hemorheology and microcirculation 64, 213-222 (2016).
4. Cintra, M. B., Ricz, H., Mafee, M. F. & Santos, A. C. d. Magnetic resonance imaging: dynamic contrast enhancement and diffusion-weighted imaging to identify malignant cervical lymph nodes. Radiologia brasileira 51, 71-75 (2018).
5. Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 45, 228-247 (2009).
6. Sourbron, S. P. & Buckley, D. L. On the scope and interpretation of the Tofts models for DCE‐MRI. Magnetic resonance in medicine 66, 735-745 (2011).